Abstract
In this study, we developed a column-switching high-performance liquid chromatography (HPLC) method with fluorescence detection for the analysis of vitamin K. Column-switching is accomplished by changing the direction of flow using a switching valve with a set time program. Using this method, three vitamin K, phylloquinone (PK), menaquinone-4 (MK-4), and menaquinone-7 (MK-7), were separated and identified with high sensitivity, and impurities were eliminated. This method was used to determine the vitamin K content in meat, fish meat, snails, bivalves, sea urchins, seaweeds, vegetables, tea, soy products, milk products, and supplements. The results showed that chicken showed the highest content of MK-4 (15.35 ± 0.35 μg/100 g), matcha showed the highest content of PK (3069.66±80.10 μg/100 g), and dried natto showed the highest content of MK-7 (3997.57±79.42 μg/100 g). This method can also be used to analyze vitamin K in supplements and pharmaceuticals. The results of this study revealed that different manufacturers add different types of vitamin K to their commercial supplements and infant formulas. The developed method provides highly reproducible and quantitative results and allows for the rapid analysis of the three vitamin K types. Thus, the method developed in this study may aid the sequential analysis of vitamin K in different samples to assess food nutrients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin K is effective in blood coagulation (Dahlbäck 2000) and bone metabolism (Seibel and Robins 1997), and prevents arteriosclerosis (Theuwissen et al. 2014) and osteoporosis (Koshihara et al. 2003). Juanola-Falgarona et al. (2014) recommended vitamin K intake because an increase in its dietary is associated with a reduced risk of cardiovascular disease, cancer, or all-cause mortality. In Japan, osteoporosis prevention and treatment guidelines recommend 250–300 μg/day of vitamin K to maintain bone health (Japan Osteoporosis Society 2015). In addition, the health benefits of vitamin K had been reported to be correlated with improved brain function (Ferland 2012), reproductive function (Ito et al. 2011), osteoarthritis (Oka et al. 2009), rheumatoid arthritis (Okamoto et al. 2007), insulin resistance (Yoshida et al. 2008), diabetes (Beulens et al. 2010), and cancer (Nimptsch et al. 2008; Juanola-Falgarona et al. 2014).
Vitamin K is a fat-soluble vitamin that occurs naturally as vitamin K1: phylloquinone (PK) and vitamin K2: menaquinone (MK). MK has 11 homologues, depending on the number of isoprene side chains (4-14) and is denoted as menaquinone-n (MK-n). Among them, MK-4 and MK-7 were the most abundant. PK is found in leafy green vegetables and seaweeds, MK-4 is found in animal products, and MK-7 is found in natto (fermented soybean).
High-performance liquid chromatography (HPLC) has been used to obtain information on the vitamin K levels in foods and supplements, and several analyses of vitamin K levels have been reported. Ostermeyer and Schmidt (2001) reported that vitamin K analysis comprises three chromatographic steps: the preliminary purification of crude lipid extracts from silica cartridges, semi-preparation using normal-phase HPLC, and analysis using reversed-phase HPLC fluorometric detection with post-column chemical reduction. Indyk et al. (2021) extracted lipids from samples using hexane, digested glycerides with lipase to remove the most abundant lipid class, and analyzed vitamin K using an HPLC system. Ahmed and Mahmoud (2015) analyzed vitamin K levels in human plasma and blood serum using an HPLC-fluorescence (FL) system. However, it is difficult to analyze vitamin K by interfering with substances in seafood and meat, which contain many lipids and other unknown substances. In such cases, HPLC analysis is performed after removing interfering substances using solid-phase extraction or thin-layer chromatography. However, these methods are complex and time consuming. The column-switching method was used to remove interfering substances from the HPLC analysis. This method allows for simple and rapid analysis of the samples. Our laboratory previously reported the use of column-switching methods for analyzing biogenic amines and vitamin D in food. During biogenic amines analysis using the column-switching method, impurities were eliminated, enabling the rapid and simultaneous analysis of biogenic amines. During vitamin D analysis using the column-switching method, ergocalciferol and cholecalciferol are separated simultaneously while eliminating impurities in one step, which consists of general saponification followed by one-time HPLC analysis. This method simplifies vitamin D2 and D3 analyses in foods compared to previous methods, and the results are highly reproducible and in line with previous reports. Therefore, in this study, we developed a method for analyzing vitamin K using the column-switching method and analyzed a variety of foods (meat, fish, snails, bivalves, sea urchins, seaweeds, vegetables, tea, soy products, milk products, and supplements).
Material and Methods
Chemicals
HPLC-grade ethanol, n-hexane, 2-propanol, and methanol were purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). Phylloquinone (PK), menaquinone-4 (MK-4), and menaquinone-7 (MK-7) were purchased from Wako Pure Chemical Industries Ltd. (Tokyo, Japan). Standard solutions of vitamin K were prepared by dissolving the compounds in ethanol. All the solutions were prepared at a concentration of 1 mg/mL of the respective compound. Each of the three solutions (1 mL) was mixed with 7 mL of ethanol. These mixed solutions contained 100 μg/mL of MK-4, PK, and MK-7. Stock and mixed solutions were diluted with ethanol and used as standards. Calibration plots were constructed by analyzing the standard solutions at four concentrations of vitamin K: 25, 50, 75, and 100 ng/mL of vitamin K compounds.
Materials
Meats: chicken, pork, and beef, Fish meats: green eye (Chlorophthalmus albatrossis), cutlass (Trichiurus japonicus), flat fish (Reinhardtius hippoglossoides), and salmon (Oncorhynchus kisutch), Snails: turban shell (Turbo sazae), and abalone (Haliotis discus hannai), Bivalves: freshwater clam (Corbicula japonica), and Japanese carpet shell (Ruditapes philippinarum). Sea urchin: purple sea urchin (Heliocidaris crassispina). Seaweeds: sea lettuce (Monostroma nitidum), wakame (Undaria pinnatifida), kelp (Saccharina japonica), and sea grapes (Caulerpa lentillifera). Vegetables: shiitake mushroom (Lentinula edodes), perilla (Perilla frutescens var. crispa) and broccoli (Brassica oleracea var. italica), Chinese chives (Pisum sativum), Japanese parsley (Cryptotaenica japonica Hassk.), bean sprouts (Pisum sativum), mizuna (Brassica rapa var. nipposinica), and Japanese mustard spinach (Brassica rapa var. perviridis), Teas: matcha, black tea, oolong tea, Pu-erh tea, roasted green tea, deep steamed tea, and gyokuro, Soy products: soy sauce, natto, and dried natto, Milk products: infant formulas, and Supplements: vitamin K pills and intestinal regulators purchased from a local store. Snails: lipped periwinkle (Monodonta confuse), and top shell (Omphalius feifferi pfeifferi) were collected from Nobeoka, Japan. Bivalves: Oyster 1-4 (Crossostrea nippona) were collected from Hososima, Yamagata, Okuise, and Goto, Japan. Dried lavers (Porphyra spp.) were a gift from Ariake Fishermen’s Cooperative, Saga Prefecture, Japan. The samples were minced with a blender (Hi-Power Blender MX1200XTM, Osaka Chemical Co., Ltd., Japan) and stored at −30 °C until analysis. Capsule type of supplement was cut into surface of capsules using a cutter and the contents were collected using a spatula. Tablet type of supplement was minced with a blender (Hi-Power Blender MX1200XTM, Osaka Chemical Co., Ltd., Japan) and stored at −30 °C until analysis.
Sample Preparation
Vitamin K was extracted from each food sample according to the Analysis Manual of the Standard Tables of Food Composition in Japan, 2015 Edition (7th Revised Edition) (2023). Briefly, each sample was weighed and put in a 50 mL glass centrifuge tube, and a specified amount of an appropriate solvent was added. The samples were homogenized using a homogenizer (POLYTRON PT-MR2100; Central Science Trading Co., Ltd.). The samples remaining on the blade were rinsed in a 50 mL glass centrifuge tube a specified amount of n-hexane. The obtained solution was shaken at 250 rpm for 5 min using a shaking machine (Maker), and then centrifuged at 2500 rpm for 5 min (Table Top Centrifuge 4000; Kubota Seisakusho Co., Ltd.). After separation, a specified amount of the n-hexane layer was collected in a 15 mL glass test tube and concentrated using a centrifugal concentrator (VC-96N, Taitec Co., Ltd.). Three milliliters of ethanol was added to dissolve the extract; however, samples with higher vitamin K content were diluted with ethanol, and the extract was stored in a refrigerator at -30 °C until HPLC analysis.
For the vitamin K-containing pill, 0.1 g of sample was weighed and placed into a 50 mL glass centrifuge tube, and 10 mL of methanol was added. The obtained solution was shaken at 250 rpm for 5 min using a shaking machine and then centrifuged at 2500 rpm for 5 min. After separation, 5 mL of the methanol layer was collected in another 50 mL test tube, and 8 mL of n-hexane was added. The obtained solution was shaken at 250 rpm for 5 min and centrifuged at 2500 rpm for 5 min. After separation, 5 mL of the n-hexane layer was collected in a 15 mL glass test tube and concentrated using a centrifugal concentrator. A suitable amount of ethanol was added to the solution to dissolve it, and the extract solutions were stored in a refrigerator at −30 °C until HPLC analysis.
In the case of teas, 0.1 g of samples were weighed and placed in a 50 mL glass centrifuge tube, and 6 mL of n-hexane was added. The obtained solution was shaken at 250 rpm for 5 min using a shaking machine and then centrifuged at 2500 rpm for 5 min. After separation, 5 mL of the n-hexane layer was collected in a 15 mL test tube and concentrated using a centrifugal concentrator. A suitable amount of ethanol was added to the solution to dissolve it, and the extract solutions were stored in a refrigerator at −30 °C until HPLC analysis.
HPLC System
The vitamin K content was analyzed using an HPLC system equipped with a PU-980 HPLC pump (JASCO Corporation, Tokyo, Japan), CTO-10A column oven (40 °C), DGU–14A degasser, SIL-10ADVP autosampler, SCL-10A system controller (Shimadzu, Kyoto, Japan), L-2485 fluorescence detector (Hitachi High-Technologies Corporation, Tokyo, Japan), automatic valve unit model VA-11 (FLOM, Tokyo, Japan), and 7725i Rheodyne-sampling injector equipped with a 20 μL sample loop (Rheodyne, Rohnert Park, CA, USA).
The system comprised CAPCELL PAK C18 MG column (75 mm × 4.6 mm, 5 μm particle size; Osaka Soda Co. Ltd. Osaka, Japan) as both pre-column and analytical column A platinum-reduction column (RC-10 4.0×15 mm; Osaka Soda Co., Ltd.) was used as the reduction column. The mobile phase of the two pumps consisted of a solution (methanol:2-propanol, 60:40 [v/v]) and the flow rates was 0.5 mL/min from each pump. The fluorescence detector was set to excitation at 320 nm and emission at 430 nm. The detected data were processed using CDS-Lite ver. 5.0 (LA Soft, Inc.).
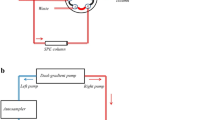
The instrumental setup is shown schematically in Fig. 1. First, the switching valve was set at position A (Fig. 1(a)). The impurities were eliminated from the pre-column. Three and a half minutes after injection, the valve was moved to position B (Fig. 1(b)). The analytical solution was injected into the pre-column, then vitamin K were migrated from the pre-column to the analytical column. After 10 min, the switching valve was changed back to position A and impurities were eliminated from the pre-column.
Method Validation
To validate the HPLC method, the intra- and inter-day precision, specificity, linearity, range, limits of detection (LOD) and quantification (LOQ), and recovery, were evaluated according to Ishimaru et al. (2019). To test the intra- and inter-day precision and specificity, 20 μL of 100 ng/mL standard solution were analyzed using HPLC. To test the linearity, range, LOD, and LOQ, analytical solutions were prepared by dilution with ethanol to an appropriate concentration, then 20 μL of each solution were analyzed using HPLC. The LOD and LOQ were defined as the concentration at which each peak was visibly separable from the baseline noise (greater than three times the noise baseline) and the concentration at which the analyte concentration was greater than 10 times the baseline noise, respectively. To evaluate recovery, fortified samples were prepared by adding a 100 μL aliquot of mixed standard solution (0: no Vitamin K, : 5 μg/mL Vitamin K compound solution, 2.5: containing 2.5 μg/mL Vitamin K compound solution,1:1 μg/mL Vitamin K compound solution, and 10:10 μg/mL vitamin K solution) to chicken fillet, green tea (Matcha), and dried Natto samples. Four pieces of samples were weighed in glass centrifuge tubes, and a 100 μL of each standard solution was added to each sample. The samples were then extracted. The recovery was estimated based on the results of this analysis.
Results and Discussion
Method Validation
A highly sensitive fluorescence detector was used for vitamin K analysis. The separation and detection by HPLC with fluorescence detection using post-column chemical reduction is relatively high sensitive, convenient and stable. Vitamin K is reduced by the platinum black -reduction column and converted into Vitamin K hydroquinones, which are highly fluorescent (Kamao et al. 2005; Shino 1988). Therefore, we used a platinum black column for the HPLC analysis of vitamin K. The platinum black -reduction column was located between HPLC analysis column and the fluorescence detector for post column reduction. When we extracted lipids from various foods and analyzed them directly, it was difficult to analyze vitamin K due to interference with substances in the case of seafood and meat, which contain many other unknown substances. In such cases, HPLC analysis is performed after removing interfering substances using solid-phase extraction or thin-layer chromatography. However, these methods are complex and time consuming. To further develop this approach, we attempted to simplify sample preparation by applying the column-switching method. Already a precedent was selected to except the interference peaks. These methods have the disadvantage of requiring two columns. However, these methods have been successfully used to concentrate analytes and remove impurities from analytical columns. Therefore, we developed an HPLC method that used a column-switching system.
First, we attempted the mobile phase of the two pumps consisting of a solution (methanol: ethanol, 60:40 [v/v]), with flow rates of 1.0 mL/min from each pump. However, in this method, ethanol was expensive, the total flow rates were 2.0 mL/min, and a burden on the column was also considered. Therefore, the mobile phase of the two pumps were changed to methanol: 2-propanol, 60:40 (v/v), with flow rates of 0.5 mL/min from each pump. Furthermore, 2-propanol is considered a suitable alternative because its polarity is similar to that of ethanol.
Second, to confirm the effect of the column-switching system, an analysis was performed without applying the switching system using a sample of sea urchins and salmon with many impurities. This system has not only an FL detector but also an ultraviolet-visible detector (220 nm) to detect impurity components other than fluorescent substances. In the analysis without the column switching system, vitamin K groups were eluted at 7–16 min; however, impurity peaks from sea urchin (Fig. 2a) and salmon (Fig. 2c) were detected. Therefore, we developed an analytical method that uses column switching to remove impurities. After examining the switching timing of the switching valve, the initial state (Fig. 1a) was set, and the sample solution was injected. Among the vitamin K compounds preliminarily separated in the pre-column, the substance analyzed in this study was eluted from the pre-column between approximately 4.0 and 8.1 min. Therefore, the substances eluted from 0 min to 3.5 min were discharged into the drain. At 3.5 min, the switching valve was switched to (Fig. 1b), and the pre-column and analytical column were connected in series to guide the substance to be analyzed in the analytical column. After 10 min, the switching valve was switched to the initial state (Fig. 1a) again to discharge the slowly eluted substances remaining in the pre-column to the drain. This method facilitated the analysis of multiple interfering peaks between 4.0 and 9.0 minutes in sea urchin (Fig. 2b) and salmon (Fig. 2d), most of which were not detected. And the peak near the end of the analysis in sea urchin and salmon were no longer detected after about 22 minutes. These results mean that no interfering substances remains in the pre and analytical-column at 25 minutes. Therefore, no interfering substances derived from the previous analysis were detected even when continuous analysis was performed. Consequently, vitamin K was separated with good specificity over a 25 min cycle. A resulted chromatogram of the mixed standard solutions (MK-4, PK, and MK-7) is shown in Fig. 3. MK-4, PK, and MK-7 were detected at 7.8, 10.0, and 14.9 minutes, respectively.
High-performance liquid chromatography (HPLC) chromatograms of sea urchins without (a) and with (b) column switching, and salmon without (c) and with (d) column switching. The orange line represents the chromatogram obtained using the UV detector. The blue line represents the chromatogram obtained using the fluorescence detector
High-performance liquid chromatography (HPLC) chromatograms of the standard solution. The numbers in the chromatograms represent the following compounds: 1, Menaquinone-4 (MK-4); 2, phylloquinone (PK); 3, Menaquinone-7 (MK-7). The arrows indicate the time points of column switching. The valve was switched in position A from 0 to 3.5 min, B from 3.5 to 10 min, and A from 10 to 25 min
To verify the precision and specificity of the developed method, the retention time (tR), peak area, capacity factor, resolution factor, and separation factor were evaluated (Table 1). The relative standard deviations (RSD) of the tRs for all vitamin K levels was < 0.04%. Therefore, vitamin K was separated with good specificity. The RSD of the peak areas yield using the method of this study was < 2.00%. Thus, this method exhibited good reproducibility. The linearity, range of detection, LOD, and LOQ are listed in Table 1. The correlation coefficients for all vitamin K levels were greater than 0.994, the method of this study indicates good linearity. The linear range varied depending on the vitamin K species. The LODs and LOQs for various vitamin K concentrations ranged from 0.234–0.501 ng/mL and 0.827–1.665 ng/mL, respectively. When the method developed in this study were compared with other reports, the LOD and LOQ of vitamin K were superior to those of previous studies that applied FL or UHPLC methods (Kim et al. 2023; Dunlop et al. 2022). Finally, the recovery level of each vitamin K was evaluated and the results are shown in Table 2. The average recovery and the RSD from chicken fillets, matcha, and dried natto, all the different vitamin K analyzed were 77.28%–107.84% and 2.71%–17.14%, respectability. These indicate that the developed method has acceptable accuracy for determining vitamin K levels in foods. A typical chromatogram of a food sample is shown in Fig. 4. Figure 4(a) shows the analysis result of chicken fillets, where MK-4 was detected in approximately 8 min; (b) is the analysis result of matcha, where PK was detected in approximately 10 min; and (c) is the analysis result of dried natto, where MK-7 was detected in approximately 15 min.
Vitamin K Contents of Foods
We investigated the vitamin K content in foods using the method developed in this study (Table 3). Chicken fillet, pork, and beef contained 15.35 ± 0.35, 5.67 ± 0.58, and 10.72 ± 1.57 μg/100 g of MK-4, respectively. Dunlop et al. (2022) reported that chicken fillets, pork, and beef contain MK-4, with chicken showing the highest value. This result was consistent with our data analysis.
Among the fishes, salmon showed high levels of MK-4 (8.86 ± 0.41 μg/100 g) and PK (2.79 ± 0.10 μg/100 g), this is considered to that salmon was aquaculture and originates from the food. Among the shells, snails showed higher PK values than bivalves. Abalone and turban shells, which are commonly eaten snails in Japan, contain high levels of vitamin K. Abalone can be divided into the muscle and liver as edible parts, and the vitamin K content was analyzed separately. The muscle and liver showed 0.73 ± 0.25 and 64.14 ± 19.63 μg/100 g of PK, respectively. Based on these results, we concluded that the vitamin K contents of the muscle and liver differed substantially. The turban shells also contained high levels of vitamin K (33.78 ±3.02 μg/100 μg of PK), however, the turban sells could not be divided into muscle and midgut line like a abalone’s. If turban shells can be separated into muscle and midgut as in abalone, the midgut must have a high PK content. The purple sea urchin also contained high levels of vitamin K (150.27 ± 8.25 μg/100 g of PK). In the present study, we analyzed only the gonads as edible parts of sea urchins. Fat-soluble vitamins in fish are more abundant in the liver than in the muscle (Dewailly et al. 2011). The midgut and gonads of shellfish contain numerous lipids (Frouin et al. 2007). Therefore, fat-soluble vitamins such as vitamin K are thought to be more abundant in the liver and gonads than in muscle. In the present study, the seaweeds contained high levels of PK because they were plant-based. Therefore, snails and purple sea urchins that feed on seaweed have high PK levels.
All vegetables and tea contained only PK, and dark-green leafy vegetables had a relatively higher PK content than light-colored vegetables. This is because PK is biosynthesized in the chloroplasts of plants, and there is a positive correlation between PK and chlorophyll (Eugeni Piller et al. 2011). In the present study, broccoli contained PK (91.97 ± 6.42 μg/100g). Damon et al. (2005) reported that the PK content of broccoli is 231 μg/100 g. In the present study, Chinese chive contained PK (145.28 ± 31.98 μg/100 g) in the present study. Kim et al. (2023) reported that the PK content of Chinese chives was 190.03 ± 1.42 μg/100 g. Matcha (3069.66 ± 80.10 μg/100 g), roasted green tea (568.03 ± 5.79 μg/100 g), deep steamed tea (223.99 ± 6.66 μg/100 g), and gyokuro (598.33 ± 28.55 μg/100 g), which are Japanese teas, have the characteristic of unfermented. Conversely, black tea (266.73 ± 4.32 μg/100 g), oolong tea (265.22 ± 15.05 μg/100 g), and pu-erh tea (219.41 ± 3.86 μg/100 g) have the characteristic of being fermented teas. Vitamin K content was higher in unfermented tea than in fermented tea, suggesting that fermentation may have resulted in vitamin K loss.
Natto 1, natto 2, and dried natto contained 331.27 ± 28.80, 674.83 ± 3.15, and 3997.57 ± 79.42 μg/100 g of MK-7, respectively. Natto and dried natto showed high levels of MK-7, which is biosynthesized by microorganisms such as Bacillus natto (Tarvainen et al. 2019). Tarvainen et al. (2019) and Jensen et al. (2022) reported that the MK-7 content in natto were 901.72 μg/100 g and 980 μg/100 g. There was a difference in MK-7 content between natto 1, natto 2, and other studies, suggesting that the MK-7 content differs depending on the product. However, soy sauce does not contain all vitamin K, which is consistent with the results of Tarvainen et al. (2019). Soy sauce, similar to natto, is a fermented soybean food that does not contain vitamin K. These results indicate that vitamin K in natto is not derived from the soybeans themselves but is produced only by Bacillus natto.
Although vitamin K has been artificially added to infant formulas by manufacturers, the ingredient label lists only the total amount of vitamin K. When we analyzed three commercial infant formulas in the present study, No. 1 infant formula contained MK-4 (6.12 ± 0.47 μg/100 g) and PK (6.49 ± 0.84 μg/100 g), No. 2 infant formula contained MK-4 (8.33 ± 0.69 μg/100 g), PK (1.28 ± 0.21 μg/100 g), and MK-7 (8.97 ± 3.65 μg/100 g), and No. 3 infant formula contained only MK-7 (251.12 ± 8.64 μg/100 g). The results indicate that each manufacturer added different types of vitamin K to the infant formula.
The analysis of three commercially available adjusted vitamin K Supplements in this study showed that No. 1 contained only MK-7 (3145.28 ± 876.93 μg/100 g) and No. 2 contained only MK-4 (678.74 ± 38.37 μg/100 g). Moreover, it was speculated that all vitamin K in the pill 1 was added with MK-7 derived from natto and pill 2, was added to MK-4. Intestinal regulators included PK (14.41 ± 2.74 μg/100 g) and MK-7 (4.36 ± 3.24 μg/100 g), which are thought to be derived from the raw plant material and Bacillus subtilis natto powder. The results indicated that each manufacturer added different types of vitamin K to the infant formula supplement.
Conclusions
In this study, three vitamin K compounds were separated and detected by column-switching HPLC. A column-switching system allowed us to evaluate vitamin K levels and remove impurities. Thus, using the method developed in this study, the analysis of vitamin K can be executed with good reproducibility, preventing column deterioration and having shorter total analysis time cycles than methods using solid-phase extraction or thin-layer chromatography. Furthermore, our method is applicable to the determination of vitamin K content in various foods and may be useful for continuous analysis of vitamin K in large numbers of samples to assess food quality. This method can be used to analyze vitamin K not only in foods, but also in supplements and pharmaceuticals. The results of the present study elucidated that manufacturers can add different types of vitamin K to commercial supplements and infant formulas.
Data Availability
The authors declare that data supporting the findings of this study are available in this article.
References
Ahmed S, Mahmoud AM (2015) A novel salting-out assisted extraction coupled with HPLC-fluorescence detection for trace determination of vitamin K homologues in human plasma. Talanta 144:480–487. https://doi.org/10.1016/j.talanta.2015.07.007
Analysis Manual of Standard Tables of Food Composition in Japan, 2015 Edition (7th Revised Edition) (2023). Web site of Office for Resources, Policy Division Science and Technology Policy Bureau, Japan. https://www.mext.go.jp/a_menu/syokuhinseibun/1368931.htm. Accessed 25 Augst 2023.
Beulens JWJ, van der ADL, Grobbee DE, Sluijs I, Spijkerman AMW, van der Schouw YT (2010) Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care 33:1699–1705. https://doi.org/10.2337/dc09-2302
Dahlbäck B (2000) Blood coagulation. Lancet 355:1627–1632. https://doi.org/10.1016/S0140-6736(00)02225-X
Damon M, Zhang NZ, Haytowitz DB, Booth SL (2005) Phylloquinone (vitamin K1) content of vegetables. J Food Comps Anal 18:751–758. https://doi.org/10.1016/j.jfca.2004.07.004
Dewailly E, Rouja P, Schultz E, Julien P, Tucker T (2011) Vitamin A intoxication from reef fish liver consumption in Bermuda. J Food Prot 74:1581–1583. https://doi.org/10.4315/0362-028X.JFP-10-566
Dunlop E, Jakobsen J, Jensen MB, Arcot J, Qiao L, Cunningham J, Black LJ (2022) Vitamin K content of cheese, yoghurt and meat products in Australia. Food Chem 397. https://doi.org/10.1016/j.foodchem.2022.133772
Eugeni Piller L, Besagni C, Ksas B, Rumeau D, Bréhélin C, Glauser G, Kessler F, Havaux M (2011) Chloroplast lipid droplet type II NAD(P)H quinone oxidoreductase is essential for prenylquinone metabolism and vitamin K1 accumulation. Proc Natl Acad Sci USA 108:14354–14359. https://doi.org/10.1073/pnas.1104790108
Ferland G (2012) Vitamin K and the nervous system: an overview of its actions. Adv Nutr 3:204–212. https://doi.org/10.3945/an.111.001784
Frouin H, Pellerin J, Fournier M, Pelletier E, Richard P, Pichaud N, Rouleau C, Garnerot F (2007) Physiological effects of polycyclic aromatic hydrocarbons on soft-shell clam Mya arenaria. Aquat Toxicol 82:120–134. https://doi.org/10.1016/j.aquatox.2007.02.005
Indyk HE, Gill BD, Wei S, Harvey L, Woollard DC (2021) Quantitation of vitamin K in milk products by pre-column reduction HPLC–fluorescence. Food Anal Methods 14:984–988. https://doi.org/10.1007/s12161-020-01922-0
Ishimaru M, Muto Y, Nakayama A, Hatate H, Tanaka R (2019) Determination of biogenic amines in fish meat and fermented foods using column-switching high-performance liquid chromatography with fluorescence detection. Food Anal Methods 12:166–175. https://doi.org/10.1007/s12161-018-1349-0
Ito A, Shirakawa H, Takumi N, Minegishi Y, Ohashi A, Howlader ZH, Ohsaki Y, Sato T, Goto T, Komai M (2011) Menaquinone-4 enhances testosterone production in rats and testis-derived tumor cells. Lipids Health Dis 10:158. https://doi.org/10.1186/1476-511x-10-158
Japan Osteoporosis Society, Prevention and Treatment Guidelines 2015. Web site of Japan Osteoporosis Society. http://www.josteo.com/ja/guideline/index.html. Accessed 25 Aug 2023
Jensen MB, Rød KE, Švarc PL, Oveland E, Jakobsen J (2022) Vitamin K (phylloquinone and menaquinones) in foods – Cost-effective quantification by LC-ESI-MS/MS. Food Chem 385. https://doi.org/10.1016/j.foodchem.2022.132672
Juanola-Falgarona M, Salas-Salvadó J, Martínez-Gonzaĺez MA, Corella D, Estruch R, Ros E, Fitó M, Arós F, Gómez-Gracia E, Fiol M, Lapetra J, Basora J, Lamuela-Raventós RM, Serra-Majem L, PintóX MMA, Ruiz-Gutiérrez V, Fernández-Ballart J, Bulló M (2014) Dietary intake of vitamin K is inversely associated with mortality risk. J Nutr 144:743–750. https://doi.org/10.3945/jn.113.187740
Kamao M, Suhara Y, Tsugawa N, Okano T (2005) Determination of plasma vitamin K by high-performance liquid chromatography with fluorescence detection using vitamin K analogs as internal standards. J Chromatogr B 816:41–48. https://doi.org/10.1016/j.jchromb.2004.11.003
Kim HJ, Shin J, Kang Y, Kim D, Park JJ (2023) Effect of different cooking method on vitamin E and K content and true retention of legumes and vegetables commonly consumed in Korea. Food Sci Biotechnol 32:647–658. https://doi.org/10.1007/s10068-022-01206-9
Koshihara Y, Hoshi K, Okawara R, Ishibashi H, Yamamoto S (2003) Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J Endocrinol 176:339–348. https://doi.org/10.1677/joe.0.1760339
Nimptsch K, Rohrmann S, Linseisen J (2008) Dietary intake of vitamin K and risk of prostate cancer in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg). Am J Clin Nutr 87:985–992. https://doi.org/10.1093/ajcn/87.4.985
Oka H, Akune T, Muraki S, En-yo Y, Yoshida M, Saika A, Sasaki S, Nakamura K, Kawaguchi H, Yoshimura N (2009) Association of low dietary vitamin K intake with radiographic knee osteoarthritis in the Japanese elderly population: dietary survey in a population-based cohort of the ROAD study. J Orthop Sci 14:687–692. https://doi.org/10.1007/s00776-009-1395-y
Okamoto H, Shidara K, Hoshi D, Kamatani N (2007) Anti-arthritis effects of vitamin K2 (menaquinone-4) − a new potential therapeutic strategy for rheumatoid arthritis. FEBS J 274:4588–4594. https://doi.org/10.1111/j.1742-4658.2007.05987.x
Ostermeyer U, Schmidt T (2001) Determination of vitamin K in the edible part of fish by high-performance liquid chromatography. Eur Food Res Technol 212:518–528. https://doi.org/10.1007/s002170000262
Seibel MJ, Robins SP (1997) Serum undercarboxylated osteocalcin and the risk of hip fracture. J Clin Endocrinol Metab 82:717–718. https://doi.org/10.1210/jcem.82.3.3847
Shino M (1988) Determination of endogenous vitamin K (phylloquinone and menaquinone-n) in plasma by high-performance liquid chromatography using platinum oxide catalyst reduction and fluorescence detection. Analyst 113:393–397. https://doi.org/10.1039/an9881300393
Tarvainen M, Fabritius M, Yang B (2019) Determination of vitamin K composition of fermented food. Food Chem 275:515–522. https://doi.org/10.1016/j.foodchem.2018.09.136
Theuwissen E, Magdeleyns EJ, Braam LAJLM, Teunissen KJ, Knapen MH, Binnekamp IAG, Van Summeren MJH, Vermeer C (2014) Vitamin K status in healthy volunteers. Food Funct 5:229–234. https://doi.org/10.1039/c3fo60464k
Yoshida M, Booth SL, Meigs JB, Saltzman E, Jacques PF (2008) Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am J Clin Nutr 88:210–215. https://doi.org/10.1093/ajcn/88.1.210
Funding
Open access funding provided by University of Miyazaki. No funding was received to assist with the preparation of this manuscript.
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanaka, R., Tanaka, R. Development of vitamin K analysis method using column switching high-performance liquid chromatography method and analysis results of various food items for vitamin K content. Food Anal. Methods (2024). https://doi.org/10.1007/s12161-024-02643-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12161-024-02643-4