Abstract
Aluminium (Al) is the third most common element in the Earth’s crust and occurs naturally in drinking water and agricultural products, and humans are consequently exposed to the element from dietary sources. A tolerable weekly intake of 1 mg/kg has been established by the European Food Safety Authority (EFSA); however, no maximum levels for aluminium in foodstuffs have so far been established in the European Union (EU) legislation. Official food control requires validated methods for the determination of aluminium. Acid digestion assisted by microwaves is the main sample preparation technique used for the determination of aluminium, usually in combination with atomic spectrometry for quantification. In the present study, different parameters in the digestion step were investigated including test portion, digestion temperature, the reagent used and duration of the digestion to assess the aluminium extraction. The presented work is following up on an observation from a proficiency test (PT) on trace elements (including aluminium) in cocoa powder organised in 2020 by the European Union Reference Laboratory for metals and nitrogenous compounds in feed and food (EURL-MN), where the participant results for aluminium showed an unexpectedly large variation. In addition to the PT material, different certified reference materials were included in the present study, and the results highlighted that the temperature and reagent used are the most critical parameters to obtain a satisfactory sample digestion prior to aluminium determination. Based on the obtained results, it is recommended to digest food samples with a mix of ultrapure water and nitric acid for 25 min at a temperature of at least 240 °C with a mix of HNO3 and H2O to achieve satisfactory microwave-assisted digestion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminium (Al) is the most abundant metal in the crust of the Earth (8.3% (w/w)) (Greenwood and Earnshaw 1997). In nature, Al is mainly found in its oxide form due to its strong affinity to oxygen. Al is released, redistributed and accumulated in water, air and biota due to natural processes such as acid rains and rock weathering (Crisponi et al. 2012; Squadrone et al. 2021). This leads Al to occur naturally and ubiquitously in drinking water and agricultural products. In aqueous systems, Al can be found as complexes, such as inorganic monomeric, amorphous and polynuclear species (Botté et al. 2022; Ščančar and Milačič 2006). Ocean acidification linked to climate change would affect the bioavailability of Al and would increase its ecotoxicological effects on aquatic ecosystems (Botté et al. 2022). Humans, in general, are mainly exposed to Al through the consumption of food and drinking water as well as from the use of consumer products and pharmaceuticals (EFSA 2008). There are currently no maximum levels in the European Union (EU) for Al content in foodstuffs. The European Food Safety Authority (EFSA) established a TWI (tolerable weekly intake) value of 1 mg Al/kg bw/week based on combined findings in animal studies showing embryotoxicity, neurotoxicity and reproductive toxicity (EFSA 2008). The mean weekly dietary exposure in the general European population was estimated to be in the range of 0.2 to 1.5 mg Al/kg bw, while the exposure was up to 2.3 mg Al/kg bw/week for toddlers (1.5–4.5 years). This TWI may be exceeded by a part of the EU population through dietary exposure (EFSA 2008; Stahl et al. 2018). The uses of Al as a food additive and in food contact materials are regulated in the EU through Commission Regulation 1333/2008 on food additives and Commission Regulation 10/2011 on plastics and articles intended to come into contact with food, respectively. In 2013, the EU published guidelines for food contact materials made of metal and stated that a specific release limit (SRL) of 5 mg/kg of foodstuff is reasonably achievable (Council of Europe 2013).
Official food control requires validated methods for the determination of Al. The European Committee for Standardization (CEN) initially published a European standard (EN) describing a procedure for the pressure digestion of foodstuffs intended for the determination of trace elements in food (CEN 2014). This procedure is not specific to Al and proposes the use of nitric acid and, if needed, hydrochloric acid, hydrogen peroxide and even hydrofluoric acid for the digestion. Recently, specific ENs for the determination of Al by either inductively coupled plasma-mass spectrometry (ICP-MS) (EN 17264:2019) (CEN 2019a) or inductively coupled plasma-optical emission spectrometry (ICP-OES) were published (EN 17265:2019 (CEN 2019b)). In these ENs, it is stated that in most cases, using only nitric acid for the sample digestion is sufficient to determine the complete Al content, with an advice to include the addition of water in case of foodstuff samples with a low water content. In the ENs, it is, however, stated that the proposed procedure may in case of the presence of poorly soluble Al compounds lead to lower findings. This is related to the presence of silicates in the sample matrix that can form poorly dissolvable complexes with Al as reported previously (Oliva et al. 2003; Sapkota et al. 2005; Smodiš and Bleise 2007). Similarly, in another study (Loeschner et al. 2018), authors reported a higher concentration of Al in noodles following digestion with a mixture of nitric acid, hydrogen peroxide and hydrofluoric acid compared to a digestion with nitric acid only and ascribed this finding to the presence of Al silicates present in particulate form.
The aim of the present work was to investigate the influence of individual digestion parameters such as test portion size, maximum temperature, reagents and duration on the Al recovery in a range of foodstuffs.
Materials and Methods
Proficiency Test on Al in Cocoa
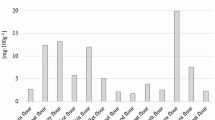
A proficiency test (PT) was organised in 2020 by the European Union reference laboratory on metals and nitrogenous compounds in feed and food (EURL-MN) with cocoa powder as a test item. The sample material was purchased in an online shop and consisted of two bags of cocoa powder of 1 kg each. Cocoa powder has in previous studies been identified as a potential high Al-containing foodstuff (Chekri et al. 2019; Stahl et al. 2018). The contents of both bags were manually mixed in a cleaned plastic bucket (approximately 10 L) with a plastic spoon for 10 min. The material was then distributed into 100 plastic containers each containing approximately 15 g of material and subsequently stored at room temperature. Homogeneity and stability studies were performed by the National Food Institute, Technical University of Denmark and were evaluated according to ISO 13528:2015 (ISO 2015) and ISO Guide 35:2017 (ISO 2017). It was shown that the material was homogeneous and stable for the duration of the PT. A total of 28 National Reference Laboratories (NRLs) from EU and European Free Trade Association (EFTA) countries participated in the PT for the Al determination. The majority (24 of 28) of the laboratories used ICP-MS for the Al determination followed by ICP-OES (3 of 28) and electrothermal atomic absorption spectrometry (ETAAS) (1 of 28). The participants were asked to perform two or three independent analyses using the procedure used for routine analysis. A relatively large variation in the reported results for Al was observed (Fig. 1), and it was suspected that not all the Al was extracted by the sample preparation procedures used by the participating laboratories, e.g. due to the use of a too-low digestion temperature. The laboratories were therefore asked to provide detailed information on the digestion parameters used (Table S1). In order to further investigate the possible reasons for the variation of the results, additional experiments were performed as described in the following sections.
Chemicals and Samples
The standard reference materials (SRMs) 1566b (oyster tissue, 197.2 ± 6.0 mg of Al /kg), 8436 (durum wheat flour, 11.7 ± 4.7 mg of Al/kg) and 1572 (citrus leaves, 92 ± 15 mg of Al/kg) were purchased from National Institute of Standards and Technology (NIST, Gaithersburg, USA). Concentrated nitric acid (67–69% HNO3, PlasmaPure, SCP Science, Quebec, Canada) was used for the digestion and dilution. Hydrogen peroxide (30% H2O2, Suprapure, Supelco, Merck KGaA, Darmstadt, Germany) was used for the digestion. Stock solution (1000 ppm) of aluminium (Al), rhodium (Rh) and bismuth (Bi) come from PlasmaCAL (SCP Science, Baie-D’Urfé, QC, Canada). Ultrapure water (UPW) (18.2 mΩ·cm at 21.5 °C) was used for the dilution, and solution preparation was obtained using a Millipore Element apparatus (Millipore, Milford, MA, USA).
Equipment
Test portions for digestion were weighed using an analytical Sartorius GENIUS ME balance (Göttingen, Germany). A Multiwave 7000 microwave oven (Anton Paar GmbH, Graz, Austria) containing 18 quartz vials (18 mL) with plug-on lids was used for the closed-vessel digestion. All solutions were prepared in 15-/50-mL disposable polypropylene tubes (Sarstedt AG & Co, KG, Germany). The total mass fraction of Al in the digested samples was determined by inductively coupled plasma-mass spectrometry (ICP-MS) using an iCAP TQ ICP-MS (Thermo Fisher Scientific, Bremen, Germany) equipped with an ASX-560 autosampler (Teledyne CETAC Technologies, Omaha, NE, USA).
Digestion Protocol
Test portions of approximately 0.3 g were weighed into 18-mL quartz tubes prior to the addition of 1 mL of ultrapure water and 4 mL of concentrated HNO3 (67–69%). The digestion program was Ramp to 250 °C for 10 min, held at 250 °C for 20 min and cooled down for 30 min. Following the digestion, the digests were transferred to 50-mL tubes, and ultrapure water was added to the 25-mL mark and subsequently further diluted 10 times in 2% HNO3 prior to analysis. Procedural blank samples were included in all analytical sequences for quality assurance. Quartz tubes were cleaned after each digestion sequence with 8 mL of concentrated HNO3 using a program of Ramp to 250 °C for 10 min, held at 250 °C for 10 min and cooled down for 30 min and rinsed with ultrapure water.
Four parameters were assessed for the digestion: (1) test portion size, (2) maximum digestion temperature, (3) digestion reagents and (4) digestion duration. These parameters were decided based on the answers provided by PT participants (Table S1) in order to be representative of the practices in the participating NRLs. The detailed assessment is described in Table 1. Regarding digestion reagents, the different conditions are abbreviated with C1 (only 3 mL HNO3), C2 (3 mL UPW and 2 mL HNO3), C3 (2 mL H2O2 and 5 mL HNO3) and C4 (3 mL UPW, 3 mL HNO3 and 1 mL H2O2).
ICP-MS Analysis
The mass fraction of Al in the samples was determined by ICP-MS in single quadrupole mode without the use of cell gas (no gas mode). An external calibration in the concentration range of 0 to 800 µg/L with Rh and Bi as internal standards (at 1 µg/L) was used to quantify the Al concentration. Standards were prepared daily and matrix-matched with the diluted samples (2% HNO3). Instrumental configuration and operating parameters for ICP-MS analysis are provided in Table 2.
The limit of detection (LOD) and limit of quantification (LOQ), calculated as 3 and 10 times the standard deviation of the procedural blank samples, were 0.3 and 0.9 mg/kg, respectively.
Statistical Analysis
Statistical tests were performed using OriginPro, Version 2023 (OriginLab Corporation, Northampton, MA, USA). One-way ANOVA was performed using 0.05 as the significance level and Tukey’s test for comparison of means.
Results and Discussion
Proficiency Test Results
Figure 1 shows the obtained results (mass fraction of Al) from the participating laboratories in the PT on cocoa powder. The assigned value was determined as a consensus value based on the results of the participants, and the associated uncertainty is the robust standard deviation following the Algorithm A calculation principles (ISO 13528:2015).
A relatively large variation between the results (coefficient of variation > 30%) was observed. The participants were then asked to provide further information on the digestion parameters used and seventeen laboratories replied (Table S1). The following observations were made when evaluating laboratory replies and Al results:
-
Test portion: No correlation observed between Al mass fraction and test portion
-
Digestion temperature: Higher results for Al mass fraction were observed at higher digestion temperatures (Figure S1)
-
Digestion reagents: No correlation between Al mass fraction and the choice of reagents was observed
-
Digestion duration: No correlation between Al mass fraction and the digestion duration was observed
Based on these observations, a set of experiments was designed to further investigate the individual digestion parameters’ influence on the obtained results.
Influence of Microwave Digestion Parameters
Test Portion Size
Three SRMs and the PT material were analysed in order to assess the influence of the test portion on Al recovery using our standard digestion protocol (Table 1). No significant recovery differences between the test portion (ANOVA, p = 0.05) were observed for any of the SRMs (Fig. 2A). For these experiments, the maximum digestion temperature was 250 °C.
Aluminium recovery in different SRM, depending on the test portion (A), maximum digestion temperature (B), digestion reagents (C) C1 (only 3 mL HNO3), C2 (3 mL UPW and 2 mL HNO3), C3 (2 mL H2O2 and 5 mL HNO3) and C4 (3 mL UPW, 3 mL HNO3 and 1 mL H2O2) (T = 250 °C) and digestion duration (D). (T = 250 °C). Error bars represent the obtained SD (standard deviation)
Higher recovery was in general achieved for durum wheat flour compared to the citrus leaves and the oyster tissue which could be related to the lower Al concentration in this material. According to the EN standard for determination of Al in foodstuffs by ICP-MS (CEN (2019a)), it is recommended to choose the smallest possible test portion (usually around 0.2 g for dry powdered samples) depending on the homogeneity of the prepared sample. In line with this, another study recommended not to use a test portion above 0.5 g to avoid interferences during the ICP-MS analysis due to the risk of achieving an incomplete digestion and consequently a high residual carbon content (Lee et al. 2022). In this study, no significant differences were found between the recoveries obtained using different test portions in the range of 0.2 to 0.5 g (Table 1), although a slightly higher recovery was seen for the smallest test portion for the citrus leaves and oyster tissue samples. These results are in agreement with the PT results, where also no correlation between the test portion and Al mass fraction was observed (Fig. 3A).
Aluminium mass fraction measured by PT participants and our laboratory (DTU) in cocoa powder (PT material) depending on the test portion (A), maximum digestion temperature (B), digestion reagents (C) C1 (only 3 mL HNO3), C2 (3 mL UPW and 2 mL HNO3), C3 (2 mL H2O2 and 5 mL HNO3) (T = 250 °C) and C4 (3 mL UPW, 3 mL HNO3 and 1 mL H2O2) and digestion duration (D) (T = 250 °C). Error bars represent the obtained SD (standard deviation)
Maximum Digestion Temperature
The results of the experiments on varying maximum digestion temperatures are shown in Fig. 2B. The recovery obtained for oyster tissue samples appears to be lower than the recoveries obtained for citrus leaves and durum wheat flour when the maximum temperature was below 220 °C. Higher maximum digestion temperatures significantly increased the Al recovery for the oyster tissue (from 45 to 94%) and the durum wheat flour (from 75 to 106%). In contrast, the temperature did not affect the Al recovery as much for citrus leaves, except at 140 °C where a lower recovery (69%) was observed. The higher recovery increase for oyster tissue could possibly be explained by this SRM having a higher Al mass fraction (197.2 mg/kg) compared to the other samples, which consequently would require higher energy to dissolve all the Al. Furthermore, the Al mass fraction in the oyster tissue is close to the level (200 mg/kg) reported in the CEN standard for which digestion temperatures above 220 °C are recommended instead of 200 °C to achieve satisfactory dissolution of Al (CEN 2019a).
For the oyster tissue, recoveries were generally satisfactory (> 80%) when using temperatures above 220 °C. The Al recoveries for citrus leaves and durum wheat flour were satisfactory at digestion temperatures above 180 °C but significantly higher (> 95%) from 240 °C for durum wheat flour. This may be explained by the presence of Al silicates in the durum wheat sample, which consequently need high temperature to fully dissolve the Al. Regarding the PT material (Fig. 3B), temperature is the only digestion parameter where participants’ results showed a correlation with Al recovery. Experiments in our laboratory also confirmed a higher recovery when increasing the digestion temperature (from 107 to 197 mg/kg). The impact of digestion temperature on Al recovery was previously reported (Min Lao et al. 2023) but only within a lower temperature range (150 to 190 °C). As the temperature is correlated to the Al mass fraction, the following experiments (Digestion reagents and Digestion duration) were performed at two different digestion temperatures: 200 °C (recommended temperature by CEN 2019a) and 250 °C (temperature used in our routine analysis).
Digestion Reagents
The results of the experiments on the use of different digestion reagents are shown in Fig. 2C. For these experiments the maximum digestion temperature was 250 °C. The lowest recoveries (72–88%) were observed when using condition C1 (HNO3). Incomplete digestion using nitric acid alone was also reported in the literature for several elements in peat, plant samples and turmeric (Sapkota et al. 2005; Zeiner et al. 2022). These results suggest that the use of nitric acid alone should be avoided. The highest recoveries were obtained with the conditions C2 (H2O + HNO3) and C4 (H2O + HNO3 + H2O2) for the three SRM samples. The addition of H2O to dilute HNO3 is already known to improve the digestion because it leads to a regeneration of HNO3 and thereby maintains the digestion reaction cycle effectively (Bizzi et al. 2010; Lee et al. 2022). Previously, the addition of H2O2 has also been reported to improve the digestion efficiency by promoting the regeneration of HNO3 (Lee et al. 2022). Our recovery results based on the reagent condition are somewhat in contrast with the digestion procedure described in the EN standard (CEN 2019a), which states that in most cases, only HNO3 is sufficient to achieve a complete dissolution of Al. Nevertheless, the results are in line with the recommendation to not use H2O2 (CEN 2019a), as it does not improve the extraction efficiency compared to when using a mix of HNO3 and H2O (C2). Similar results were obtained for the digestion of the PT material (Fig. 3C) where also higher mass fractions of Al were obtained when using conditions C2 and C4 and the lowest recovery was obtained with condition C1. Regarding the temperature (Figure S2), in every condition, the recoveries were lower (between 6 and 19% lower) for digestion conducted at 200 °C compared to digestion at 250 °C, confirming the impact of the digestion temperature used on the Al extraction efficiency. Based on these results, a mix of H2O and HNO3 is recommended for the digestion of food samples for Al determination.
Digestion Duration
The different digestion duration results are presented in Fig. 2D. For these experiments, the maximum digestion temperature was 250 °C. The duration of the digestion (20–50 min) did not have a significant impact on Al recovery for the durum wheat flour and citrus leaves. For the oyster tissue sample, increasing the duration of the digestion improved the Al recovery from 77% (20 min) to 87% (50 min). For this material, satisfactory recoveries (> 80%) were obtained for digestion durations above 25 min, which is somewhat longer than the digestion duration of 20 min recommended by the EN standard (CEN 2019a) to obtain a complete digestion in the microwave. The experiment was also performed at a digestion temperature of 200 °C (Figure S3), and for all the materials, lower recoveries were obtained, again confirming the importance of the digestion temperature. Concerning the PT material (Fig. 3D), the results showed an increase in the Al mass fraction when increasing the digestion duration from 20 min (184 mg/kg) to 50 min (211 mg/kg). At lower temperature (200 °C), the Al concentration increase was lower (from 132 to 140 mg/kg). Nevertheless, these results confirmed again the impact of digestion temperature on the Al extraction. According to these results, a digestion duration of at least 25 min is recommended to obtain a satisfactory microwave-assisted digestion.
Conclusion
In the present study, the influence of different digestion parameters including test portion, maximum digestion temperature, digestion reagents and digestion duration on the recovery of Al was investigated using different types of food samples. The experiments showed that digestion temperature and choice of digestion reagent can affect the extraction efficiency. Based on the obtained results, a digestion temperature of at least 240 °C and a digestion duration of at least 25 min using a mix of HNO3 and H2O are recommended for microwave-assisted digestion of food samples prior to Al determination by ICP-MS. These recommendations provide important additional information to the EN standard method provided by CEN and will be useful for laboratories to improve their protocol for the determination of Al in food samples by ICP-MS.
References
Bizzi CA et al (2010) Microwave-assisted digestion in closed vessels: effect of pressurization with oxygen on digestion process with diluted nitric acid. Anal Methods 2(6):734–38. https://doi.org/10.1039/C0AY00059K
Botté A, Zaidi M, Guery J, Fichet D and Leignel V (2022) Aluminium in aquatic environments: abundance and ecotoxicological impacts. Aquat Ecol 56(3):751–73. https://doi.org/10.1007/s10452-021-09936-4
[CEN] European Committee for Standardization EN 13805:2014 (2014) Foodstuffs - determination of trace elements - pressure digestion. https://www.cencenelec.eu/. Accessed 25 Apr 2024
[CEN] European Committee for Standardization. EN 17264:2019 (2019a) Foodstuffs – determination of elements and their chemical species – determination of aluminium by inductively coupled plasma mass spectrometry (ICP-MS). https://www.cencenelec.eu/. Accessed 25 Apr 2024
[CEN] European Committee for Standardization EN 17265:2019 (2019b) Foodstuffs - determination elements and their chemical species - determination of aluminium by inductively coupled plasma optical emission spectometry (ICP-OES). https://www.cencenelec.eu/. Accessed 25 Apr 2024
Chekri R et al (2019) Trace element contents in foods from the first French total diet study on infants and toddlers. J Food Compos Anal 78:108–120. https://doi.org/10.1016/j.jfca.2019.02.002
Council of Europe, and European Directorate for the Quality of Medicines & HealthCare (2013) Metals and alloys used in food contact materials and articles - a practical guide for manufacturers and regulators. https://www.edqm.eu/en/home. Accessed 25 Apr 2024
Crisponi G et al (2012) Chelating agents for human diseases related to aluminium overload. Coord Chem Rev 256(1–2):89–104. https://doi.org/10.1016/j.ccr.2011.06.013
[EFSA] European Food Safety Authority (2008) Safety of aluminium from dietary intake ‐ scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC). EFSA J 754:1–34. https://doi.org/10.2903/j.efsa.2008.754
Greenwood NN, Earnshaw A (1997) Aluminium, gallium, indium and thallium. In: Greenwood NN, Earnshaw A (eds) Chemistry of the Elements. Elsevier, pp 216–67
[ISO] International Organization for Standardization 13528:2015 (2015) Statistical method for use in proficiency testing by interlaboratory comparison. ISBN 978 0580 73566 0. (last accessed on 25/04-2024) www.iso.org. Accessed 25 Apr 2024
[ISO] International Organization for Standardization ISO Guide 35:2017 (2017) Reference materials — guidance for characterization and assessment of homogeneity and stability. www.iso.org. Accessed 25 Apr 2024
Lao M, Yong CL, Qu BZ, Jin H (2023) Development and validation of single-step microwave-assisted digestion method for determining heavy metals in aquatic products: health risk assessment. Food Chem 402:134500. https://doi.org/10.1016/j.foodchem.2022.134500
Lee J, Park YS, Lee HJ, Koo YE (2022) Microwave-assisted digestion method using diluted nitric acid and hydrogen peroxide for the determination of major and minor elements in milk samples by ICP-OES and ICP-MS. Food Chem 373:131483. https://doi.org/10.1016/j.foodchem.2021.131483
Loeschner K et al (2018) Detection and characterisation of aluminium- containing nanoparticles in Chinese noodles by single particle ICP-MS. Food Addit Contam: Part A 35(1):86–93. https://doi.org/10.1080/19440049.2017.1382728
Oliva SR, Raitio H, Mingorance MD (2003) Comparison of Two Wet Digestion Procedures for Multi-element Analysis of Plant Samples. Commun Soil Sci Plant Anal 34(19–20):2913–2923. https://doi.org/10.1081/CSS-120025216
Sapkota A et al (2005) Analytical procedures for the determination of selected major (Al, Ca, Fe, K, Mg, Na, and Ti) and trace (Li, Mn, Sr, and Zn) elements in peat and plant samples using inductively coupled plasma-optical emission spectrometry. Anal Chim Acta 540:247–56. https://doi.org/10.1016/j.aca.2005.03.008
Ščančar J, Milačič R (2006) Aluminium speciation in environmental samples: a review. Anal Bioanal Chem 386(4):999–1012. https://doi.org/10.1007/s00216-006-0422-5
Smodiš B, Bleise A (2007) IAEA quality control study on determining trace elements in biological matrices for air pollution research. Journal of Radioanalytical and Nuclear Chemistry 271(2):269–274. https://doi.org/10.1007/s10967-007-0203-4
Squadrone S et al (2021) Aluminium occurrence in plant feed from Northwestern Italy. J Trace Elem Med Biol 68:126850. https://doi.org/10.1016/j.jtemb.2021.126850
Stahl T et al (2018) Evaluation of human exposure to aluminum from food and food contact materials. Eur Food Res Technol 244(12):2077–2084. https://doi.org/10.1007/s00217-018-3124-2
Zeiner M, Šoltić M, Nemet I, Cindrić IJ (2022) Multielement determination in turmeric (Curcuma longa L.) using different digestion methods. Molecules 27(23):8392. https://doi.org/10.3390/molecules27238392
Acknowledgements
The authors would like to thank laboratory technicians Mrs. Birgitte Koch Herbst and Mrs. Yuka Omura Lund for their highly skilled laboratory assistance during the proficiency test and extended experiments. National reference laboratories are thanked for their participation in the proficiency test and for providing additional information.
Funding
Open access funding provided by Technical University of Denmark Parts of this work were included in the EURL-MN work program 2019–2020 and work program 2021–2022 funded by the European Commission.
Author information
Authors and Affiliations
Contributions
L.G.: investigation, conceptualising, experiment design, sample preparation and analysis, writing - original draft; H.A.: project administration, experimental design, supervision, writing – review and editing; K.L.: experimental design, supervision, writing – review and editing; J.J.S.: project administration, experimental design supervision, writing – review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Givelet, L., Amlund, H., Loeschner, K. et al. Aluminium Determination in Foodstuffs by ICP-MS: Influence of Microwave Digestion Parameters on the Recovery. Food Anal. Methods 17, 1038–1045 (2024). https://doi.org/10.1007/s12161-024-02628-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-024-02628-3