Abstract
A simple, effective, and ligandless liquid-liquid microextraction (LLME) procedure based on the decomposition of hydrophobic deep eutectic solvents (HDES) was developed for the separation and pre-concentration of chromium (VI) ions in spinach leaves, before the determination by flame atomic absorption spectrophotometry. In the proposed study, the first stage involved the leaching of chromium (VI) from spinach leaves with 0.1 M Na2CO3, and in the second stage, chromium (VI) extract was preconcentrated with the LLME procedure using a DES prepared from the combination of DL-menthol and formic acid as a chelating agent and extraction solvent. The DES decomposed in an aqueous donor phase resulting in the dispersion of menthol and extraction of Cr (VI) ions. Under optimal experimental conditions, the limits of detection (LOD) and quantification (LOQ) were 0.63 and 2.1 µg L−1, respectively. The relative standard deviation (RSD) was less than 7%, and the pre-concentration factor (PF) was found to be 31.25. The accuracy of the present methodology was tested by recovery experiments. The greenness of the developed method was assessed using three quantitative green metrics tools: Analytical Eco-scale, AGREE, and AGREEprep, with only Analytical Eco-scale qualifying the proposed method as green.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Contamination by heavy metals has become a worldwide environmental problem due to anthropogenic and natural sources. They are harmful to humans and the environment because of their toxicity and carcinogenicity (Aigbe and Osibote 2020). The oxidation state of a metal determines its potential risk and benefits; therefore, an accurate determination of each species is of great importance (Abkenar et al. 2010). Chromium is one of the most common heavy metals found in nature. It occurs mainly in two stable oxidation states, namely (+3) and (+6). Hexavalent chromium is a public concern because of its toxic effects on humans, animals, plants, and microorganisms (Alemayehu et al. 2011). Chromium (VI) can easily enter groundwater and soil through natural and anthropogenic sources and poses a serious threat to the health of human beings via its entry into the food cycle (Ahmed et al. 2017). Therefore, it is of great importance to determine the concentration of Cr(VI) in a variety of food and biological samples to protect human safety. Atomic absorption spectrometry (AAS), whether with flame or electrothermal atomization, remains one of the most established and widely used techniques for the determination of Cr in various matrices (Zeeb et al. 2013, Nguyen et al. 2022, Goodarzi et al. 2022, Yan et al. 2023). However, sample preparation is necessary to reduce the influence of the matrix effects as well as to preconcentrate the analytes of interest due to their low content in the sample (Shirani et al. 2019, Andruch et al. 2022). Liquid-liquid microextraction (LLME) is a miniaturized version of the conventional liquid-liquid extraction method (LLE) and has attracted significant attention as a sample preparation technique because of its simplicity of operation, short extraction time, high preconcentration factors, and low analysis costs (Sajid and Alhoosani 2018, Zhu et al. 2018). However, halogenated organic solvents are the most used extraction solvents in LLME methods. Even though they are used in minute volumes, they are toxic, volatile, flammable, and environmentally unsustainable (He et al. 2022). Nowadays, the trend in sample preparation methods has been focused on replacing environmentally unsustainable solvents with new solvents that comply with the concept of green chemistry such as ionic liquids (ILs) and deep eutectic solvents (DESs) (Ribeiro et al 2015). Ionic liquids are a group of organic salts with low melting points below 100 °C, negligible vapor pressure, low flammability, and designability (Dai et al. 2013). However, the synthesis is complex and costly, and they are not biodegradable (Quin et al. 2020).
Deep eutectic solvents (DESs) are analogs of ILs and share some of the unique physicochemical properties with ILs, such as low volatility, nonflammability, and designability, and were first proposed by Abbott and coworkers (Abbott et al. 2003). They are prepared by heating two or more components that function as a hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD) at a certain mole ratio through hydrogen bond interactions to form a eutectic mixture with a melting point lower than that of each component (Smith et al. 2014). They have several advantages over ILs and traditional organic solvents such as simplicity of preparation, nontoxic, biocompatibility and biodegradability, and low production cost (Florindo et al. 2020). Examples of the use of DES in LLME for the extraction of metal ions (Karimi et al. 2015, Pourmohammad et al. 2020, Shishov et al 2020a, b, Ragheb et al. 2021, Pinheiro et al 2021, Shamsipur et al. 2022) have been reported in the literature demonstrating that they are becoming more acceptable as green alternative solvents. In 2018, a dispersive liquid-liquid microextraction method based on the in situ decomposition of a DES in the aqueous phase was published for the first time by Shishov and coworkers (Shishov et al. 2018). In their method, a DES made of tetrabutylammonium bromide (TBABr) and formic acid was used as the dispersive agent, and octanol was the extraction solvent. The aqueous sample phase was injected into an extraction mixture of dispersive solvent (DES) and extraction solvent, which resulted in the decomposition of the DES, and dispersion of the extraction solvent in the aqueous sample phase was observed resulting in fast extraction of analytes into extraction solvent droplets (Shishov et al. 2018). Shishov and coworkers developed another method in 2019 where a DES made of TBABr and heptanol was injected into an aqueous sample resulting in the dissolution of its water-soluble component and simultaneous dispersion of the organic phase and extraction of hydrophobic analytes (Shishov et al. 2019). Since then, LLME methods based on the decomposition of DES in the aqueous phase have been utilized for the preconcentrating of organic compounds in the liver (Shishov et al. 2020a, b), organic analytes in honey (Nemati et al. 2021), antifungal drugs in biological samples (Ezoddin et al. 2022), and metal ions in beverages and vegetable oil (Shishov et al. 2020a, b, Shishov et al. 2022). In this work, we report on the utilization of a DES prepared by mixing DL-menthol as an HBD and formic acid as HBA in liquid-liquid microextraction based on in situ decomposition of DES for a ligand-less extraction and preconcentration of Cr(VI) ions in spinach sample by flame atomic absorption spectrophotometry. Several parameters affecting the proposed procedure were investigated such as the selection of DES, DES molar ratio, DES volume, sample pH, the volume of sample, and salting out effect. Furthermore, the greenness of the proposed method was assessed using quantitative green metrics tools, namely, Analytical Eco-Scale (AES), AGREE, and AGREEprep.
Materials and Methods
Reagents and Materials
All reagents and solvents used in this study were of analytical grade. Formic acid (FA), acetic acid (AA), trichloroacetic acid (TCAA), hydrochloric acid, sodium hydroxide, and DL menthol were all obtained from Fluka Biochemica-Sigma-Aldrich (Steinheim, Germany). The chromium standard (1000 µg mL−1) was obtained from (Merck, Germany). The working standard solutions were prepared by appropriate dilutions of the stock solution with double distilled water to obtain proper concentrations. Ten millimeter centrifuge tubes with a conical bottom were used for extraction, and a 1-mL Hamilton syringe was used for removing the organic phase.
Samples
The spinach samples (swiss chard) used in this study were acquired at local supermarkets in Nairobi, Kenya. The samples were dehydrated in the oven at 80 °C for 24 h to remove excess water. Following that, a blender was used to grind the samples. Prior to usage, the samples underwent screening. For all the optimization and validation studies, spinach samples (spiked or nonspiked) were utilized.
Instruments
Absorbance measurements were performed with a Shimadzu AA6200 Flame Atomic Absorption Spectrophotometer (Kyoto, Japan) containing a 100-mm burner head and a deuterium lamp for background correction. The flame was generated with a mixture of acetylene and air with flow rates of 2.3 and 15 L min−1, respectively. A chromium hollow-cathode lamp operated at a current of 10 mA, wavelength of 357.9 nm, and spectral resolution of 0.7 nm was used. The pH measurements of sample solutions were carried out using a Metrohm pH meter model 654 (Herisau, Switzerland). A Hettich centrifuge, model ROTOFIX 32A (Kirchlengern, Germany), was used for phase separation. A laboratory hot plate from Gerhardt (Konigswinter, Germany) was used for all the heating.
Preparation of DL-Menthol-Based DES
The synthesis of DES was carried out by mixing DL menthol (as a common HBA) with different organic acids, namely formic acid, acetic acid, and trichloroacetic acid (as HBDs) in a 10-mL screw-cap tube with the desired molar ratio. Following the addition of the aforementioned components, the contents of the screw-cap tube were sealed and placed in a water bath at 80 °C with constant stirring until a homogeneous liquid formed.
Pretreatment of Spinach Samples
The chromium (VI) in spinach was determined according to a method developed by Panichev et al. 2005. The sample was prepared by weighing 0.25 g (dry weight) of spinach leaves, spiking with total chromium standard at 10 µg L−1, allowing it to dry, and thereafter transferred into a 100-mL glass beaker. Twenty-five milliliters of 0.1 mol L−1 Na2CO3 was added, and the contents of the beaker were boiled for 15 min on a hot plate before being allowed to cool. After cooling, the sample was filtered through a Whatman no. 1 filter paper and diluted to a final volume of 25 mL with double distilled. It should be noted that the aqueous Cr(VI) solution obtained in this section was used as a donor phase for preconcentration by the LLME procedure.
Liquid-Liquid Microextraction Procedure
Eighty microliters of DES made from DL-menthol and formic acid at a ratio of 2:1 was transferred into a 10-mL centrifuge tube followed by rapid injection of 2.5 mL of aqueous spinach sample solution (obtained from the “Pretreatment of Spinach Samples” section) previously adjusted to pH 3 followed by 100 mg of NaCl, this resulted in DES decomposition. The tube was then centrifuged for 5 min at 4000 rpm, and the top organic phase was easily collected with a syringe and transferred to another clean dry test tube and diluted with 0.50 mL of 0.5 M HNO3. The resulting solution was directly injected into the flame atomic absorption spectrophotometer, for the determinations.
Statistical Analysis
The data obtained in this study were subjected to an analysis of variance (ANOVA) for testing the linearity significance and the calculation of correlation coefficient (R) and coefficient of determination (r2); the LODs and LOQs were calculated from linearity. Data was processed using the computer program Excel 2016 (Microsoft Office®). The level of statistical significance was set at p < 0.05.
Results and Discussion
An LLME method based on the in situ decomposition of DES was developed in this study for the extraction and preconcentration of Cr(VI) before determination by flame atomic absorption spectrophotometer. A pretreatment step is the most crucial step in the determination of hexavalent chromium in plant samples. The chosen method’s experimental conditions should be able to quantitatively solubilize Cr(VI) while also avoiding the interconversions between Cr(VI) and Cr(III) (Seby and Vacchina 2018).In 2005, Panichev and coworkers demonstrated that chromium (VI) compounds in plants can be leached using 0.1 M Na2CO3 (Panichev et al. 2005). The method has since been used for determination of Cr(VI) in soil and plants (Mandiwana et al. 2007), different teas (Mandiwana et al. 2011a, b), medicinal plants (Owolabi et al. 2016), and bread together with breakfast cereals (Mathebula et al. 2017). In the proposed study, the first step was the pretreatment of the previously dried and ground spinach leaves with 0.1 M solution of Na2CO3 for the leaching of Cr(VI). The 2.5 mL of this extract was then used as the donor phase in the LLME procedure for the preconcentration of Cr(VI). In the LLME procedure, the deep eutectic solvent served as the dispersive agent, chelating agent, and extraction solvent. The aqueous Cr(VI) extract was directly added to the synthesized DES (DL-menthol: formic acid), resulting in the decomposition of the hydrophilic and hydrophobic components of DES due to the strong interactions between the aqueous phase and the formic acid. Formic acid served as a dispersive agent while the menthol served as an extraction solvent and chelating agent. Parameters such as the sample pH, composition of DES, the effect of DES ratio, volume of DES, sample volume, and salting out effect were studied and optimized to obtain the best conditions for the proposed method. All the optimization studies were carried out in triplicate using 2 mL of the spinach extract previously spiked with 10 µg L−1. One variable at a time (OVAT) was adopted for all the optimization studies, and the analytical results were expressed as the mean value ± standard deviation.
Optimization of LLME Procedure
Effect of DES Composition
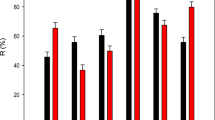
In the LLME method based on DES decomposition, a DES as an extraction solvent is critical and should be able to decompose when in contact with aqueous media. To facilitate DES decomposition and leaching out into the water phase, a hydrophobic DES synthesized by the combination of a hydrophilic and a hydrophobic precursor is a requirement (Florindo et al. 2017, Nemati et al. 2021). Various eutectic mixtures incorporating menthol as HBA with different HBDs have already been reported in the literature as hydrophobic DES due to its low water solubility (Ribeiro et al. 2015, Fan et al. 2020, Ortega-Zamora et al. 2020, Yena and Row 2020). In this study, DL menthol was incorporated as HBA in the DES synthesis together with three different hydrophilic organic acids namely: formic acid (FA), trichloroacetic acid (TCAA), and acetic acid (AA) at a 1:1 mole ratio. Among the DESs synthesized, the absorbance signal increased in the following HBD order, formic acid > trichloroacetic acid > acetic acid (Fig. 1). Despite TCAA having the highest acidity (pka value of 0.66 among the HBDs used), formic acid exhibited the highest absorbance. This could be attributed to the commercial formic acid’s high water content (Díaz-Álvarez and Martín-Esteban 2022) which might have aided in the DES decomposition. The DES created with formic acid as the HBD was chosen as the optimum for further studies.
Effect of DES Mole Ratio
The ratio between HBA and HBD is one of the important factors as it influences the physicochemical properties and extraction ability of a DES (Zhang et al. 2012). To obtain the optimum ratio of the extraction solvent, different mole ratios (1:1, 1:2, 1:3, 3:1, and 2:1) of DL menthol: formic acid were investigated. As can be seen in Fig. 2, the DES prepared at mole ratios where the proportion of menthol increased up to 2 gave the highest absorbance, and there was a decrease when the ratio of the acid was increased. This is most likely owing to the positively charged menthol being more available to interact the Cr(Vi) ions.The DL menthol: formic acid at 2:1 was chosen for further studies.
Effect of Sample pH
The pH of the aqueous solution before extraction is an important parameter that affects the analyte-DES complex formation and the extraction of the analytes into especially for ionizable analytes such as Cr(VI) (Sorouraddin et al. 2020, Pourbakhshi 2021). It should be noted that no complexing agent was used in this study because the DES also functioned as a chelating agent. For this purpose, the pH of the aqueous phase (Cr(VI extract) was adjusted in the pH range of 2–8 by adding 1 M HCl or NaOH solution. As seen in Fig. 3, the absorbance increased up to pH 3 and decreased up to pH 6. This is because at lower pHs Cr(VI) is present as HcrO4−, which is the negatively charged form of Cr(VI) (Fenti et al. 2020), and it has also been shown that the DL-menthol molecule acquires a net positive charge as an HBA in the presence of organic acids as HBDs (Paul et al. 2023) thus resulting in the electrostatic interaction between HCrO4− and DL-menthol molecule and thereby making the transfer of Cr(VI) from the aqueous phase into the DL-menthol. The results demonstrated in Fig. 3 show that the absorbances increase from pH 2 to 3 and decrease at pHs higher than 5. Similar observations were reported by other researchers (Shi et al. 2020, Pourbakhshi et al. 2021). In their studies, they observed that the extraction of Cr (VI) was the best in the pH ranges from 2 to 5. The pH of the sample was optimum at 3.
Effect of DES Volume
The DES volume plays a very crucial role as it affects the preconcentration or dilution of the extracted analyte (Shishov et al. 2019). To examine the effect of DES volume, a series of volumes from 50 to 200 µL were evaluated. As shown in Fig. 4, an increase in the volume of the extraction solvent led to the dilution effect. Different extraction volumes would result in various volumes of the upper phase. The use of 80 µL of DES was chosen as the optimum in subsequent experiments.
Salting Out Effect
The addition of salt has often been shown to reduce the solubility of the analytes in aqueous environments and promote mass transfer into the organic phase, which leads to an increase in the extraction efficiency of the target analytes (Farajzadeh et al. 2018). In studies where the hydrophilic component of a DES was a salt for example, tetrabutylammonium bromide (Shishov et al 2018, Shishov et al. 2019), the salt facilitated the salting out effect and promoted mass transfer between the two phases. For this reason, the salting out effect was examined by studying the absorbance in the presence of various amounts of NaCl (0 to 100 mg). While NaCl is a non-expensive salt, it is important to reduce the amounts of reagents while keeping good extraction efficiencies. It should be noted that we did not exceed 100 mg because we intended to use as little material as possible. As can be seen in Fig. 5, increasing the amount of salt causes an increase in absorbance; thus, 100 mg NaCl was used.
Effect of Sample Volume
The sample volume can influence the extraction efficiency and preconcentration factor (PF) (Shishov et al. 2019). The volume ratio of organic: aqueous phases is important to reach a higher enrichment factor and extraction efficiency for a lower concentration of the analytes from the larger sample volume (Pourbakhshi et al. 2021). The sample volume was varied from 1.0 to 2.5 mL. The optimum volume was achieved at 2.5 mL as shown in Fig. 6. It should be noted that we did not go further than 2.5 mL because we wanted to use less material. Thus, a sample volume of 2.5 mL was selected as the optimum.
Analytical Performance of the PROPOSED METHOD
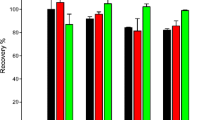
The analytical performance of the proposed method was investigated using linearity, precision, accuracy, the limit of detection (LOD), the limit of quantification (LOQ), recoveries, and preconcentration factor (PF). Matrix-matched standards were used to mitigate any matrix interferences. The linearity was evaluated by using a 5-point matrix-matched calibration curve, and this was in the range of 15 to 250 µg L−1. The coefficient of determination (r2) was 0.9996 and the correlation coefficient (r) was 0.9999, indicating good linearity. The linearity significance was evaluated using ANOVA, where a relationship between absorbance and concentration is expected. From Table 1 it can be seen that Regression SS > Residual SS (0.00043683 2 > 1.37704 x 10−07), Regression MS > Residual MS (0.000436832 > 6.88522 x 10−08), and F > Significance F (6344.496622 > 0.00015758), thus indicating a relationship between absorbance and concentration. The limit of detection (LOD) was estimated from the linear regression based on the formula xlod = 3sy/x/b where sy/x is the standard error of the regression and b is the slope. Similarly, the limit of quantification was calculated using the expression LOQ = 10sy/x/b. The LOD and LOQ obtained were 0.63 and 2.1 µg L−1 respectively. Recovery experiments were used to assess the accuracy of the proposed method. Three different concentrations (25, 50, and 100 µg L−1) were added to spinach samples and subjected to the proposed method. The recoveries obtained ranged from 96.32 to 104% at all spiked concentration levels (Table 2) and PF was 31.25. The repeatability of the method was tested by measuring three repeated extractions of spinach sample solution spiked at the 25 µg L−1 concentration level on the same day, and the reproducibility was tested over 3 consecutive days, the percent relative standard deviation was 5.3% and 6.7%, indicating good repeatability.
Assessment of Method Greenness
The greenness of the method was evaluated using three quantitative green metrics tools: Analytical Eco-scale, AGREE, and AGREEprep. The details, which include the advantages and disadvantages of the proposed metrics tools, can be found elsewhere in the literature (Sajid et al. 2022). Briefly, the Analytical Eco-scale approach provides quantitative information regarding the greenness of the analytical method in terms of the type and amount of reagents and solvents, instrument energy, occupational hazards, and waste generation. To determine the final eco-scale score, penalty points are allocated to each parameter, and the total penalty points are subtracted from 100 (Gałuszka et al. 2012). The AGREE metric, on the other hand, is based on 12 principles of green analytical chemistry, which are translated into a unified scale ranging from zero to one (Pena-Pereira et al. 2020). The AGREE results are presented in a pictogram with a zero to one scale indicating the overall score in the center and the individual scores for each GAC principle outside. The red-yellow-green color scale represents the methodological performance in each of the twelve assessment principles, while the dimension of each principle’s associated sector represents the weight of each principle. Very recently, Pena-Pereira et al. have suggested AGREEprep as an excellent indicator for the comprehensive sustainability assessment that focuses on sample preparation (Pena-Pereira et al. 2022). This metric tool is based on ten effect categories, which are then recalculated into sub-scores on a 0–1 scale. Each section in a pictogram is a different color, ranging from red to green. The AGREE categories are as follows: (a) in situ sample preparation; (b) use safer solvents and reagents; (c) target sustainable, reusable, and renewable materials; (d) minimize waste; (e) minimize sample, chemical and material amounts; (f) maximize sample throughput; (g) integrate steps and promote automation; (h) minimize energy consumption; (i) choose the greenest possible post-sample preparation configuration for analysis; and (j) ensure operator safety. The evaluation process generates a pictogram with a score in the center that summarizes the method’s overall greenness. Table 3 demonstrates that our proposed method received a score of 72 on the Eco-scale, indicating that it is an acceptable green method. The developed method, on the other hand, received an overall AGREE score of 0.49. Although the proposed methodology has the fewest red color in AGREE, it is not considered green, and hence, it represents a negative ecological impact on the environment. According to the literature, an AGREE score of at least 0.6 needs to be attained for the method to be considered green (Ferreira et al. 2022). The criteria to focus on to improve the method are in situ measurement, waste generation minimization, multiple analyte analysis, energy consumption minimization, and reducing the use of toxic solvents. Table 3 also includes the results of the AGREEprep of the proposed method. The method evaluated was based on liquid phase microextraction based on in situ decomposition of deep eutectic solvent for the determination of chromium (VI). Ex situ extraction was performed using DES synthesized from DL-menthol and formic acid, both of which are considered sustainable or renewable. The method used non-hazardous 100 mg NaCl and 0.1 M Na2CO3, which were counted as waste because of their contact with the sample. The sample also contained toxic and corrosive NaOH and HCl for pH adjustment, as well as HNO3 used for final dilution before analysis. The sampling procedure was manual and consisted of three steps. With a sample throughput of 4 per hour, the energy demand was estimated to be 148 Wh. The total amount of waste included pure solvents, mixtures, and filter paper. The final determination technique was FAAS, and more than four hazards were identified resulting from the toxicity of the solvents utilized. With a final score of 0.34, analysis using the AGREEprep metric showed that the applied method did not satisfy the requirements for the method to be considered green. This method can be made green by opting for greener alternatives for criteria 1, 2, 7, and 10, which corresponds to favoring in situ sample preparation, using safer solvents and reagents, integrating steps and promoting automation, and ensuring safe procedures for the operator. In conclusion, the proposed method, as assessed by AGREE and AGREEprep tools, was found to be unsustainable, and it is characterized by a range of different GAC criteria that highlight negative environmental problems, including health and safety. Only AES qualified the proposed method as green. The disagreement between these tools may be attributed to their vast difference in the criteria used to evaluate the proposed method.
Conclusions
A liquid-liquid microextraction based on in situ decomposition of the deep eutectic solvent method was developed and optimized for separation, preconcentration, and determination of Cr(VI) in spinach leaves before flame atomic absorption spectrophotometry detection. The developed method allows for the preconcentration and determination of Cr(VI) in spinach samples at µg L−1 levels with good reproducibility and accuracy. The proposed method was compared with other microextraction methods reported in the literature for preconcentration and the determination of Cr(VI) in food and other samples (Table 4). The relative standard deviation was comparable to that of other approaches. In contrast to other approaches, the PF was lower. The suggested method is superior to other to other approaches in a number of ways. For example, it uses Na2CO3, a common reagent that is readily available in most laboratories to separate Cr(VI). It is also ligandless. The greenness of the procedure was assessed utilizing a range of green metric tools.
Data Availability
Data will be made available upon request.
References
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V (2003) Novel solvent properties of choline chloride/urea mixtures. Chem Commun 1:70–1
Abkenar SD, Hosseini M, Dahaghin Z, Salavati-Niasari M, Jamali MR (2010) Speciation of chromium in water samples with homogeneous liquid-liquid extraction and determination by flame atomic absorption spectrometry. Bull Korean Chem Soc 31(10):2813
Ahmed S, Tuj-Zohra F, Khan SH, Hashem A (2017) Chromium from tannery waste in poultry feed: a potential cradle to transport human food chain. Cogent Environ Sci 3:1312767
Aigbe UO, Osibote OA (2020) A review of hexavalent chromium removal from aqueous solutions by sorption technique using nanomaterials. J Environ Chem Eng 8(6):104503
Alemayehu E, Thiele-Bruhn S, Lennartz B (2011) Adsorption behaviour of Cr(VI) onto macro and micro-vesicular volcanic rocks from water. Sep Purif Technol 78:55–61
Andruch V, Halko R, Tuček J, Płotka-Wasylka J (2022) Application of deep eutectic solvents in atomic absorption spectrometry. Trends Anal Chem 147:116510
Dai Y, van Spronsen J, Witkamp G, Verpoorte R, Choi YH (2013) Natural deep eutectic solvents as new potential media for green technology. Anal Chim Acta 766:61–68
Díaz-Álvarez M, Martín-Esteban A (2022) Preparation and further evaluation of l-menthol-based natural deep eutectic solvents as supported liquid membrane for the hollow fiber liquid-phase microextraction of sulfonamides from environmental waters. Adv Sample Prep 4:100047
Ezoddin M, Abdi K, Behnamipour S, Javadi MHS (2022) Air-assisted in situ deep eutectic solvent decomposition followed by the solidification of floating organic droplets-liquid-liquid microextraction method for extraction of azole antifungal drugs in biological samples prior to high-performance liquid chromatography. J Sep Sci 45:1757–1765
Fan Y, Wu H, Cai D, Yang T, Yang L (2020) Effective extraction of harmine by menthol/anise alcohol-based natural deep eutectic solvents. Sep Purif Technol 250:117211
Farajzadeh MA, Mohebbi A, MogaddamMRA Davaran M, Norouz M (2018) Development of salt-induced homogenous liquid–liquid microextraction based on iso-propanol/sodium sulfate system for extraction of some pesticides in fruit juices. Food Anal Methods 11:2497–2507
Fenti A, Chianese S, Iovino P, Musmarra D, Salvestrini S (2020) Cr(VI) sorption from aqueous solution: a review. App Sci 10:6477
Ferreira SS, Brito TA, Santana APR, Guimarães TGS, Lamarca RS, Ferreira KC, Lima Gomes PCF, Oliveira A, Amaral CDB, Gonzalez MH (2022) Greenness of procedures using NADES in the preparation of vegetal samples: comparison of five green metrics. Talanta Open 6:100131
Florindo C, Branco LC, Marrucho IM (2017) Development of hydrophobic deep eutectic solvents for extraction of pesticides from aqueous environments. Fluid Phase Equilib 448:135–142
Florindo C, Monteiro NV, Ribeiro BD, Branco LC, Marrucho IM (2020) Hydrophobic deep eutectic solvents for purification of water contaminated with bisphenol-A. J Molec Liq 297:111841
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J (2012) Analytical Eco-scale for assessing the greenness of analytical procedures. Trends Anal Chem 37:61–72
Goodarzi L, Bayatloo MR, Chalavi S, Nojavan S, Rahmani T, Azimi SB (2022) Selective extraction and determination of Cr(VI) in food samples based on tandem electromembrane extraction followed by electrothermal atomic absorption spectrometry. Food Chem 373:131442
He S, Tang W, Row KH (2022) Determination of thiophanate-methyl and carbendazim from environmental water by liquid-liquid microextraction (LLME) using a terpenoid-based hydrophobic deep eutectic solvent and high-performance liquid chromatography (HPLC). Anal Lett 55(8):1235–1248
Hemmatkhah P, Bidari A, Jafarvand S, Hosseini MRM, Assadi Y (2009) Speciation of chromium in water samples using dispersive liquid–liquid microextraction and flame atomic absorption spectrometry. Microchim Acta 166(1–2):69–75
Karimi M, Dadfarnia S, Shabani AMH, Tamaddon F, Azadi D (2015) Deep eutectic liquid organic salt as a new solvent for liquid-phase microextraction and its application in ligandless extraction and preconcentraion of lead and cadmium in edible oils. Talanta 144:648–654
Mandiwana KL, Panichev N, Kataeva M, Sieber S (2007) The solubility of Cr(III) and Cr(VI) compounds in soil and their availability to plants. J Hazard Mater 147:540–545
Mandiwana KL, Panichev N, Panicheva S (2011) Determination of chromium (VI) in black, green and herbal teas. Food Chem 129:1839–1843
Mandiwana KL, Panichev N, Panicheva S (2011) Determination of chromium (VI) in black, green and herbal teas. Food Chem 129(2011):1839–1843
Mathebula MW, Mandiwanaa K, Panichev N (2017) Speciation of chromium in bread and breakfast cereals. Food Chem 217:655–659
Nemati M, Mogaddam MRA, Farazajdeh MA, Tuzen A, Khandaghi J (2021) In-situ formation/decomposition of deep eutectic solvent during solidification of floating organic droplet-liquid-liquid microextraction method for the extraction of some antibiotics from honey prior to high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1660:462653
Nguyen C-H, Le-Thi A-D, Nguyen T-N, Le-Thi H-M, Nhon-Duc Le, Do M-H (2022) Chromium (VI) analysis in effluents using liquid liquid extraction coupled with flame atomic absorption spectrometry. Malays J Anal Sci 26(2):283–294
Ortega-Zamora C, Gonzàlez-Sàlamo J, Hernandez-Sànchez C, Hernandez-Borge J (2020) Menthol-based deep eutectic solvent dispersive liquid−liquid microextraction: a simple and quick approach for the analysis of phthalic acid esters from water and beverage sample. ACS Sustain Chem Eng 8:8783–8794
Owolabi IA, Mandiwana KL, Panichev N (2016) Speciation of chromium and vanadium in medicinal plants. South Afr J Chem 69:67–71
Panichev N, Mandiwanaa TK, Kataeva M, Sieber S (2005) Determination of Cr(VI) in plants by electrothermal atomic absorption spectrometry after leaching with sodium carbonate. Spectrochim Acta Part B 60:699–703
Paul N, Harish G, Banerjee T (2023) Stability mechanism of menthol and fatty acid based hydrophobic eutectic solvents: insights from nonbonded interactions. ACS Sustain Chem Eng 11:3539–3556
Pena-Pereira F, Wojnowski W, Tobiszewski M (2020) AGREE—analytical greenness metric approach and software. Anal Chem 92(14):10076–10082
Pena-Pereira F, Tobiszewski M, Wojnowski W, Psillakis E (2022) A tutorial on AGREEprep an analytical greenness metric for sample preparation. Adv Sample Prep 3:10025
Pinheiro FC, Aguirre MÁ, Nóbrega JA, Gonzàlez-Gallardo N, Ramón DJ, Canals A (2021) Dispersive liquid-liquid microextraction based on deep eutectic solvent for elemental impurities determination in oral and parenteral drugs by inductively coupled plasma optical emission spectrometry. Anal Chim Acta 1185:339052
Pourbakhshi Y, Heidari M, Yahaei E, Ghiyas S, Ebrahimi-Najafabad H, Bozorgzadeh E (2021) Dispersive liquid–liquid microextraction followed by solidified floating organic drop for hexavalent chromium determination: a method for occupational and environmental exposure monitoring for heavy metals. J Anal Chem 76(6):714–720
Pourmohammad M, Faraji M, Jafarinejad S (2020) Extraction of chromium (VI) in water samples by dispersive liquid–liquid microextraction based on deep eutectic solvent and determination by UV–Vis spectrophotometry. Int J Environ Anal Chem 100(10):1146–1159
Qin H, Hu X, Wang J, Cheng H, Chen L, Qi Z (2020) Overview of acidic deep eutectic solvents on synthesis, properties and applications. Green Energy Environ 5:8–21
Ragheb E, Shamsipur M, Jalali F, Sadeghi M, Babajani N, Mafakheri N (2021) Magnetic solid-phase extraction using metal–organic framework-based biosorbent followed by ligandless deep-eutectic solvent-ultrasounds-assisted dispersive liquid–liquid microextraction (DES-USA-DLLME) for preconcentration of mercury (II). Microchem J 166:106209
Ribeiro BD, Florindo C, Iff LC, Coelho MAC, Marrucho IM (2015) Menthol-based eutectic mixtures: hydrophobic low viscosity solvents. ACS Sustain Chem Eng 3:2469–2477
Sajid M, Alhooshani K (2018) Dispersive liquid-liquid microextraction based binary extraction techniques prior to chromatographic analysis: a review. Trends Anal Chem 108:167–182
Sajid M, Płotka-Wasylka J (2022) Green analytical chemistry metrics: a review. Talanta 238:123046
Sèby FF, Vacchina V (2018) Critical assessment of hexavalent chromium species from different solid environmental, industrial and food matrices. Trends Anal Chem 104:54–68
Shamsipur M, Mafakheri N, Babajani N (2022) A natural deep eutectic solvent–based ultrasound-vortex-assisted dispersive liquid–liquid microextraction method for ligand-less pre-concentration and determination of traces of cadmium ions in water and some food samples. Food Anal Methods 15:1203–1213
Shi Y, Xiong D, Zhao Y, Li T, Zhang K, Fan J (2020) Highly efficient extraction/separation of Cr(VI) by a new family of hydrophobic deep eutectic solvents. Chemosphere 241:125082
Shirani M, Habibollahi S, Akbar S (2019) Centrifuge-less deep eutectic solvent based magnetic nanofluid-linked air agitated liquid–liquid microextraction coupled with electrothermal atomic absorption spectrometry for simultaneous determination of cadmium, lead, copper, and arsenic in food samples and non-alcoholic beverages. Food Chem 281:304–311
Shishov A, Volodina N, Nechaeva D, Svetlana Gagarinova S, Bulatov A (2018) Deep eutectic solvents as a new kind of dispersive solvent for dispersive liquid–liquid microextraction. RSC Adv 8:8146–38149
Shishov A, Renata Chromà R, Vakh C, Kuchà J, Simon A, Andruch V, Bulatov A (2019) In situ decomposition of deep eutectic solvent as a novel approach in liquid-liquid microextraction. Anal Chim Acta 1065:49–55
Shishov A, Terno P, Moskvin L, Bulatov A (2020) In-syringe dispersive liquid-liquid microextraction using deep eutectic solvent as disperser: determination of chromium (VI) in beverages. Talanta 206:120209
Shishov A, Gerasimov A, Nechaeva D, Volodina N, Bessonova E, Bulatov A (2020) An effervescence-assisted dispersive liquid–liquid microextraction based on deep eutectic solvent decomposition: determination of ketoprofen and diclofenac in liver. Microchem J 156:104837
Shishov A, Volodina N, Semenova E, Navolotskaya D, Ermakov S, Bulatov A (2022) Reversed-phase dispersive liquid-liquid microextraction based on decomposition of deep eutectic solvent for the determination of lead and cadmium in vegetable oil. Food Chem 373:131456
Smith EL, Abbott AP, Ryder K (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082
Sorouraddin SA, Farajzadeha MA, Dastoori H (2020) Development of a dispersive liquid-liquid microextraction method based on a ternary deep eutectic solvent as chelating agent and extraction solvent for preconcentration of heavy metals from milk samples. Talanta 208:120485
Yan J, Zhang C, Wang C, Lu D, Chen S (2023) Direct immersion dual-drop microextraction for simultaneous separation and enrichment of Cr(III) and Cr(IV) in food samples prior to graphite furnace atomic absorption spectrometry detection. Food Chem 406:134276
Yena A, Row KH (2020) Evaluation of menthol-based hydrophobic deep eutectic solvents for the extraction of bisphenol A from environment water. Anal Lett 54(9):1533–1545
Zeeb M, Ganjali MR, Norouzi P (2013) Preconcentration and trace determination of chromium using modified ionic liquid cold-induced aggregation dispersive liquid–liquid microextraction: application to different water and food samples. Food Anal Methods 6:1398–1406
Zhang Q, De Oliveira Vigier K, Royer S, Jerome F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41(21):7108–7146
Zhu S, Zhou J, Jia H, Zhang H (2018) Liquid–liquid microextraction of synthetic pigments in beverages using a hydrophobic deep eutectic solvent. Food Chem 243:351–356
Acknowledgements
The authors would like to thank the Department of Chemistry at the University of South Africa and the Department of Food Nutrition and Dietetics, at Kenyatta University, Kenya for providing the laboratory facilities.
Funding
Open access funding provided by University of South Africa. This study was funded by National Research Foundation grant #138250 and the University of South Africa.
Author information
Authors and Affiliations
Contributions
All the authors made a significant contribution and reviewed and approved the final draft of the manuscript. DM: the conceptualizing, design of the study, data acquisition and interpretation, and drafting of the manuscript; TAM: data interpretation for green assessment studies; HNN, SD, and MMN: the conceptualizing and critical revision of the manuscript for important intellectual content and approval of the version of the manuscript to be published
Corresponding author
Ethics declarations
Competing Interests
D. Moema declares no conflict of interest. TA. Makwakwa declares no conflict of interest. H.N. Nyambaka declares no conflict of interest. S. Dube declares no conflict of interest. MM Nindi declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moema, D., Makwakwa, T., Nyambaka, H.N. et al. Method Development and Optimization of Liquid-Liquid Microextraction Based on the Decomposition of Deep Eutectic Solvent for the Determination of Chromium (VI) in Spinach: Assessment of the Greenness Profile Using Eco-scale, AGREE, and AGREEprep. Food Anal. Methods 17, 464–474 (2024). https://doi.org/10.1007/s12161-024-02583-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-024-02583-z