Abstract
The nutritional and economic value of oils is affected by its antioxidant properties linked to their total phenolic content (TPC). The analysis of antioxidants such as phenols may be affected by the lipophilic character of the media. Therefore, the accurate determination of TPC in oils deserves particular attention. This research has tested the effect of the alkaline medium in the quantification of TPC in extra virgin olive oil (EVOO) and olive pomace oil (OPO) using the Folin-Ciocalteu method. Seven different EVOO and OPO oils were used to gain representativity and study matrix effects in the analysis. Among the alkaline conditions assayed, the Folin-Ciocalteu method led to maximum TPC in EVOOs samples using 7.5% Na2CO3, whereas 35% Na2CO3 showed better characterization of TPC in OPOs than milder basic conditions. Therefore, when analyzing olive oil products, adjusting the alkaline media in the Folin Ciocalteu assay is important for optimal TPC determination. TPC analyses via Folin-Ciocalteu with different media was supported with antioxidant activities determined with reference assays: 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid cation radical and 2,2-diphenyl-1-picrylhydrazyl radical.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
There are health benefits associated with the consumption of polyphenols; for instance, their intake reduces diabetes, hypertension, neurodegenerative cardiovascular disease, and obesity and favors longevity (Anderson and Nieman 2016; Manach et al. 2004). The Mediterranean diet, recognized as highly beneficial, includes regular consumption of varied fresh fruits and vegetables, and olives and olive oil are main components in it (Anderson and Nieman 2016; Young and Woodside 2001). Olive oil is rich in polyphenols that are known to protect from tissue insult from their ability to scavenge free radicals (Shahbaz et al. 2021; Visioli et al. 2002). The total phenolic content (TPC) is a parameter used to inform about the quality of olive oil (Lechhab et al. 2022). Extra virgin olive oil (EVOO) is a superior category of olive oil that has been obtained solely by means of mechanical methods. It has lower content of hydrolytic or oxidative decomposition products than refined olive oil and it has high market value. There is a gap in knowledge in relation to the numerous cultivations, pre- and post- production, and categorization factors leading to EVOO (Lechhab et al. 2022), and it is susceptible to be misclassified or even adulterated. The International Olive Council (IOC/T.15/NC No 3/Rev.15/2019) details chemical and sensorial properties that define EVOO, Olive Pomace Oil (OPO), and other oil categories.
EVOO’s beneficial properties are associated to monounsaturated fatty acids (oleic acid among them accounts for up to 80% of the total lipid composition) which are stable to oxidation, unsaponifiable compounds (e.g., sitosterols, squalene, triterpenes), α-tocopherol, and polyphenols (Boskou 2015; Yubero-Serrano et al. 2019). TPC is an important parameter in the physico-chemical characterization of EVOO. VOO is a category of oil that has not been refined and includes some hydrolytic and oxidative decomposition compounds (Yubero-Serrano et al. 2019). OPO is obtained from extracting and refining oil from pressed olive residues (water and olive stone, pulp, skin, and oil). In OPO, the remnant oil left during olive oil production can be blended with VOO (Boskou 2015). Accurate analytical methods to determine oil properties are needed since different categories of oil and derivates have distinct health, organoleptic, and economic value. Furthermore, methods to process and preserve oils are under study and these need to measure their effectivity in preserving the properties of the starting material (Ainsworth and Gillespie 2007; Díez-Betriu et al. 2022; Baccouri et al. 2022).
High-throughput analytical approaches for the characterization of olive oils and derivatives are needed. Visible-near infrared spectroscopy (Violino et al. 2022) and FTIR (Tahir et al. 2019; Scatigno and Festa 2022), both supported with statistical treatment of the spectra, are recent developments in the area. However, the analysis that they offer has limited identification power in complex oil mixtures and lacks quantitative information. In contrast, the quantification of phenols by liquid chromatography provides specific quantitative information but requires equipment that is not in every laboratory (e.g., from cooperatives producing oil), is labor intensive, and falls short when wanting to quantify the global phenolic composition of the oils. There is current methodology to quantify a relatively high number of targeted phenols (e.g., 23 in EVOO) thanks to the use of complementary techniques (UV–Vis (photodiode array), fluorescence, and mass spectrometry (Lechhab et al 2021)). Furthermore, comprehensive 2D liquid chromatography can lead to the identification of greater number of phenols (e.g., 83 in food spices) (Arena et al 2022) in comparison with 1D chromatography. The strength of a multidimensional approach is the provision of qualitative characterization; however, the quantification of all the analytes identified is not practical with it. Chromatographic equipment equipped with the abovementioned detectors is generally available in specialized laboratories.
Hence, the global analysis of phenols with spectrophotometric analysis is a very desirable approach. TPC is commonly quantified using Folin-Ciocalteu assay (Singleton and Rossi 1965; Singleton et al. 1999). With such method, phenolic compounds are oxidized in alkaline medium with molybdotungstophosphoric heteropolyanion reagent (Folin-Ciocalteu reagent), which is yellowish, to form a mixture of blue molydotungstophosphate that can be measured spectrophotometrically (Castro-Alves and Cordenunsi 2015; Fuentes et al. 2012). Other well-established methods that characterize antioxidant properties are the DPPH· and ABTS·+ assays. These measure reactions that involve electron transfer from antioxidants to reagents. A research question that will be addressed in this work is whether redox reactions in TPC, ABTS·+, and DPPH· assays are affected by the hydrophobic character or compounds from the sample media. This is because, generally, these assays are used in the characterization of aqueous solutions (Busquets et al. 2006) and even grains (Schendel 2019).
The Folin-Ciocalteu method has limited selectivity as the reduction of its main reagent can be triggered by the oxidation of nonphenolic compounds such as metals (Fe2+ and Mn2+), I− and SO32−, ascorbic acid, thiols, nucleotides (guanine), tryptophan, trioses (glyceraldehyde and dihydroxyacetone), vitamins, and proteins (Singleton et al. 1999; Everette et al. 2010; Schendel 2019). Other experimental aspects that may affect the quantification of phenols are the order in which the reagents are added, reaction time, temperature of the reaction, and the concentration of methanol in the final mixture to be analyzed spectrophotometrically (Fuentes et al. 2012). A solution to overcome the limited selectivity of the Folin-Ciocalteu assay was to carry out partial least squares regression modeling from spectra obtained from diluted oil samples in hexane with UV spectroscopy (210–340 nm) (Fuentes et al. 2012). Clearing substances from the matrix interfering in the analysis would also increase the accuracy of the assay (Castro-Alves and Cordenunsi 2015). The accuracy of the determination of phenols is also affected by the different responses that polyphenols have in the assay (Singleton et al. 1999; Schendel 2019). The significance of this study is the assessment of an aspect that may challenge the accuracy of the widely used Folin-Ciocalteu assay for the analysis of phenols and its sensitivity: the media used in the assay for the analysis of non-hydrophilic samples such as oils.
Materials and Methods
Chemicals and Materials
Olive oils of different categories EVOO and OPO were purchased from Packages Mall (Lahore, Pakistan). Brand names for both sample categories were Borges, Mundail, Italia, Sasso, Meuolivia, La espranula, and Romoli olio. Solvents used in sample treatment were methanol and hexane, both of analytical quality and were acquired from Sigma-Aldrich (St. Louis, USA) (purity 99.9%). Sodium carbonate (Na2CO3, assay > 99.5%) was purchased from Merck (Darmstadt, Germany) and gallic acid (assay > 97.5%) was obtained from May and Baker (Wandsworth, England) and Folin-Ciocalteu reagent was obtained from Sigma Aldrich (St. Louis, USA). All chemicals used for antioxidant assays were obtained from Sigma-Aldrich (St. Louis, USA).
Liquid–Liquid Extraction of Phenolic Compounds
Phenolic compounds were extracted from oil with the method proposed by Kelebek et al. (2017) (Kelebek et al. 2017). Briefly, oil samples (4 g) were mixed with hexane (2 mL) and extracted with methanol/water (80:20, 4 mL) in a single step. After shaking, the solution mixture was centrifuged at 5500 rpm for 15 min (Kelebek et al. 2017); to separate the top layer enriched with phenols, this layer is called polyphenolic extract (PE). The procedure was carried out in triplicate. Calibration curves with gallic acid (ranging between 0.05 and 0.6 mM) were prepared with different proportions of Na2CO3 (7.5%, 20%, and 35%) in water. Absorbance measurements were carried out after 1 h at 725 nm with a UV 5100B UV/Vis spectrophotometer (Metash, Shanghai, China).
Determination of Total Phenolic Content and Antioxidant Activity Assays
The determination of the TPC was carried out with the Folin-Ciocalteu method (Singleton and Rossi 1965; Singleton et al. 1999) with modifications, where 3 equivalent portions of 1 mL from PE from all 14 samples (from 7 branded oils with OPO or EVOO categories) separated for further analysis. To study the effect of alkaline medium on the transfer of electrons from phenolates to phosphomolybdic and phophotungstic acid, each portion was separately mixed with 2.5 mL of Folin-Ciocalteu reagent (diluted by 1:10 in deionized water) and then kept in the dark for 5 min. After 5 min of incubation, 1 mL of 7.5% Na2CO3 was added in portion A of all 14 categories; 20% Na2CO3 in portion B; and 35% Na2CO3 in portion C. All tubes were incubated in the dark for 2 h. Then, the absorbance at two different wavelengths 725 nm and 765 nm was measured (Ballus et al. 2015). Each assay was carried out in triplicate. Independent calibration curves of gallic acid in 7.5%, 20%, and 35% Na2CO3 were prepared for the quantification of samples with the same proportion of Na2CO3.
An approach to determine the antioxidant activity was with the DPPH· method (Gargouri et al. 2013) with modifications in the dilutions used. Briefly, in the analysis of the antioxidant activity, or the analysis of the % radical scavenging activity (% RSA) of polyphenols in oilive oils, three different dilutions of each PE (30, 60, and 90 120 µl/mL) were prepared in methanol. An aliquot (0.5 mL) of the diluted PE was mixed with 2.5 mL of DPPH· working solution in methanol at 6·10−5 M. The mixture obtained was shaken and kept for 30 min in the dark while frequently monitoring reduction in absorbance during discoloration of the extract, which indicated the reduction of DPPH·, radical at 515 nm. After 30 min, the decrease in absorbance was recorded (Gargouri et al. 2013) and the % RSA from the DPPH· assay was calculated using Eq. 1, where A DPPH· corresponds to the Absorbance of the DPPH· working solution, and AE corresponds to the absorbance of the extract with DPPH· solution.
For the ABTS·+ assay, equal proportions of 7 mM ABTS·+ and 2.45 mM potassium persulfate (K2S2O8) were mixed. The mixture was allowed to stand at room temperature in the dark for 16 h before using it for producing ABTS·+ radicals. After 16 h, the solution was diluted with methanol to reach absorbance values of 0.7 ± 0.01 at 734 nm. Four dilutions of PE (30, 60, and 90 120 µl PE/mL final extract) were prepared in methanol. An aliquot (0.5 mL) of the diluted PE was taken from each concentration and 3.9 mL of ABTS·+ solution was added to each extract. The mixture was shaken vigorously and allowed to stand in dark. After 6 min, the decrease in absorbance was monitored at 734 nm and % RSA was calculated for the ABTS·+ assay with formula in Eq. 2, where A ABTS·+ is the absorbance of the ABTS·+ starting or control solution, and AE is the absorbance of ABTS·+ control solution containing the sample extract.
IC50, defined as the concentration of the sample that inhibits ABTS·+ or DPPH· by 50%, was determined with a line of best fit between (%RSA) and the volume of the fraction assayed.
Statistical Analysis
Collected raw data was analyzed with Microsoft XLStats software version 2017. Shapiro–Wilk test was used to test normality; and regression analysis was carried out with Pearson correlation coefficient. ANOVA with post hoc Tukey’s test were carried out. The values for p < 0.05 were regarded as significant and values for p < 0.001 as very significant.
Results and Discussion
Effect of Na2CO3 on TPC in Oil Samples
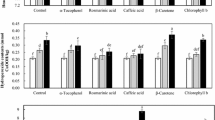
TPC was quantified after a single liquid–liquid extraction step that included hexane, methanol, and water. This solvent supported the extraction of free phenols of contrasting polarities (Castro-Alves and Cordenunsi 2015); however, phenols structurally bound to cell walls might have not been extracted with this procedure and if so, it may lead to underestimating TPC specially from OPO. The assessed TPC for the samples in the present study showed that EVOO presented higher TPC than the OPO samples (p 0.05), in all cases, under the study conditions (Fig. 1). This confirms the greater phenolic quality generally attributed to EVOO. This comparison and the quantified phenolic concentrations, expressed as gallic acid equivalents (GAEs), are displayed in Fig. 1 and reported in the Supporting Information (Table S1).
Singleton and Rossi (1965) recommended carrying out the Folin-Cicoalteu assay with ≤ 3% Na2CO3 in the final mixture to prevent the formation of precipitates. Hence, separation steps (e.g., filtration) before the spectrophotometric measurement were needed (Singleton and Rossi 1965). The content of methanol in the samples was found critical for the formation of precipitates (Cicco and Lattanzio 2011; Singleton and Rossi 1965). In this work, the hexane layer from the liquid–liquid extraction was used for the study and cloudiness or the formation of precipitates was not observed in the samples to be analyzed with UV–Vis.
When comparing EVOO and OPO with the same extraction conditions, EVOO had 4–13 times greater TPC at 7.5% Na2CO3, 2–6 times greater TPC at 20% Na2CO3, and 1–3 times greater TPC at 35% Na2CO3 in the selected range of oils (Fig. 1 and Table S1). This demonstrates that the proportion of Na2CO3 in the assay does impact significantly (p 0.05) the TPC quantified with the Folin-Ciocalteu assay (ANOVA and post hoc Turkey HSD data treatment given in Table 2S). A general trend was found in the EVOO categories: TPC7.5% Na2CO3 > TPC20% Na2CO3 > TPC35% Na2CO3. Interestingly, the general trend of concentration of phenols quantified with Folin-Ciocalteu method with different basic media in all OPO categories was the opposite than in EVOO: TPC35% Na2CO3 > TPC20% Na2CO3 > TPC7.5%. For instance, Italia OPO was a sample with maximum TPC values among all OPO categories (unlike in EVOO). For Italia OPO, TPC was 208 ± 2.41 mg gallic acid equivalent (GAE)/kg using 7.5% Na2CO3; however, TPC35% Na2CO3 presented 318 ± 1.33 mg/kg GAE. The basic media increased the abundance of phenols in their phenolate form. This form facilitates their oxidation while the Folin-Ciocalteu reagent (yellowish) gets reduced to a blue color. There are several reactions that may interfere in Folin-Ciocalteu reaction (Singleton and Rossi 1965). The change observed in the content of polyphenols quantified depending on the proportion of sodium carbonate could be caused by the change in the sensitivity for the analysis of phenols, or changes in the presence of other substances from the sample matrix that could give signal and be miss quantified as phenols, or that might affect the redox reaction. As shown in Fig. 1, given that the same alkali conditions can lead to the highest or lowest content of phenols depending on whether it is VOO or OPO, the hypothesis of matrix components interfering in the products of the reaction is likely, as opposed to enhancement of the phenolic signal in the assay which would be expected to take place regardless of the sample matrix. Alternatively, to optimizing the basicity of the extraction media, an enzymatic hydrolysis, for instance with α-amylase, followed by alkaline extraction conditions (which reduces the viscosity of the sample and enhance the extraction of phenols), both steps carried out at reaction temperature from 37 °C, might also be useful to detect greater concentrations of TPC (Castro-Alves and Cordenunsi 2015).
In this study, there was no correspondence between TPC in EVOO and OPO. This may be because the phenolic content in OPO depends on refining stages and dilution with EVOO oil. The TPC values of OPO categories were in agreement with those estimated in Algerian OPO (136.8 mg/kg GAE) (Ainsworth and Gillespie 2007). Some olive oil categories available in US presented concentrations of TOC (554–810 mg/kg GAE) (Ardo 2005) in the same order of magnitude than in this study (Table S1).
Effect of Na2CO3 on Antioxidant Activity
IC50 was determined in oils in the OPO and EVOO categories with linear regressions (Table 1). The correlation between TPC values and IC50 of each brand category is shown in Table 2. Higher polyphenolic content and high antioxidant activity are associated with low IC50 values [32]. IC50 values determined by DPPH·, ABTS·+, and TPC (7.5% Na2CO3) in samples from the OPO category were 27–68, 10–35, and 60–208 mg/kg GAE, respectively, whereas the corresponding ranges for the EVOO oils was 10–40, 6–14, and 678–919 mg/kg GAE. Hence, the broader range of values found with the TPC quantification indicates that TPC can discriminate better the different samples than the other two assays. Furthermore, TPC carried out in different alkali media was correlated in most cases with ABTS·+ and DPPH· assays (see p values in Table 2). In more detail, the antioxidant activity determined by DPPH· assay for the EVOO category of all seven brands showed very strong (TPC7.5% Na2CO3, R: − 0.963, p < 0.001); TPC20% Na2CO3, strong (R: − 0.919, p < 0.05); and TPC35% Na2CO3 weak (R: − 0.660, p > 0.05) correspondence with TPC (Table 2). In the case of antioxidant activity determined by the ABTS·+ assay for EVOO, all seven brands showed very strong (TPCB, R: − 0.954, p < 0.001); TPC7.5% Na2CO3, strong (R − 0.938, p < 0.05); and TPC35% Na2CO3, weak (R − 0.825, p < 0.05) correlation with TPC. The reaction mechanism taking place in the Folin‐Ciocalteu, ABTS·+, and DPPH· methods is a single electron transfer; thus, high correlation among their results was expected (Ballus et al. 2015).
The results in the present stay agree with previous works. According to a study conducted with Turkish EVOO from different geographical regions, the ABTS·+ method showed high correlation (R: 0.89) with TPC (Karaosmanoglu et al. 2010). In a previous study, where antioxidant activity in Spanish EVOO was determined using DPPH· and ABTS·+ assays, there was high correlation between total phenols and antioxidant capacities (Gorinstein et al. 2003). With regard to the antioxidant activity determined with the DPPH· assay for samples in the OPO category assayed under different Na2CO3 strength, TPC 35% Na2CO3 showed strong (R: − 0.929, p < 0.05) correlation and TPC 7.5% Na2CO3 and 35% Na2CO3 showed weak correlation with DPPH· IC50 (TPC 7.5% Na2CO3, R: − 0.854, p < 0.05; TPC 20% Na2CO3, R: − 0.849, p > 0.05) (Table 2). When the antioxidant activity was determined with the ABTS·+ assay and compared with TPC, TPC 20% Na2CO3 quantified for EVOO category of all seven brands showed strong correlation (R: − 0.954, p < 0.001), while TPC 7.5% Na2CO3 and TPC 35% Na2CO3 showed slightly weaker correlation (R − 0.938, p < 0.05) and (r − 0.825, p < 0.05). In previous studies, olive oils analyzed for TPC with Folin-Ciocalteu were correlated with DPPH· IC50 (R: 0.89) and ABTS·+ IC50 (R: 0.69), and with DPPH· (R: 0.72) and ABTS·+ (R: 0.76) (Laincer et al. 2014). The antioxidant capacity of the oil extracts was also significantly correlated (R: − 0.88; p < 0.0001) with the TPC (Owen et al. 2000). Hence, although the Folin-Ciocalteu reaction can be affected by components of the matrix, the correlation with antioxidant activity when changing %Na2CO3 indicates that the basicity and viscosity of the media change, and such conditions can be used to classify the oils. The best conditions for the Folin-Ciocalteu found were 7.5% Na2CO3 for characterizing EVOO samples, and 35% Na2CO3 for characterizing OPO samples.
Conclusions
The difference in TPC extracted under 7.5%, 20%, and 35% Na2CO3 for EVOO and OPO was statistically significant < 0.001 and < 0.05 respectively. Dilute alkaline media led to quantifying maximum TPC in EVOO categories and hence this media is advantageous for EVOO. In the case of OPO categories, TPC 35% Na2CO3 led to quantifying maximum TPC in OPO. This research shows that the sensitivity and accuracy of the TPC analysis in olive products carried out with the Folin Ciocalteu method improves when adjusting the alkalinity of the media.
Data Availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2:875–877. https://doi.org/10.1038/nprot.2007.102
Anderson JJ, Nieman DC (2016) Diet quality—the Greeks had it right! Nutrients 8:636. https://doi.org/10.3390/nu8100636
Ardo SA (2005) Characterization of olive oils commercially available in the United States. Dissertation, University of Maryland, College Park, USA
Arena K, Trovato E, Cacciola F, Spagnuolo L, Pannucci E, Guarnaccia P, Santi L, Dugo P, Mondello L, Dugo L (2022) Phytochemical characterization of Rhus coriaria L. extracts by headspace solid-phase micro extraction gas chromatography, comprehensive two-dimensional liquid chromatography, and antioxidant activity evaluation. Molecules 27:1727. https://doi.org/10.3390/molecules27051727
Baccouri B, Rajhi I, Theresa S, Najjar Y, Mohamed SN, Willenberg I (2022) The potential of wild olive leaves (Olea europaea L. subsp. oleaster) addition as a functional additive in olive oil production: the effects on bioactive and nutraceutical compounds using LC-ESI-QTOF/MS. Eur Food Res Technol 248(11):2809–2823. https://doi.org/10.1007/s00217-022-04091-y.
Ballus CA, Meinhart AD, de Souza Campos Jr FA, Godoy HT (2015) Total phenolics of virgin olive oils highly correlate with the hydrogen atom transfer mechanism of antioxidant capacity. J Am Oil Chem Soc 92:843-851.https://doi.org/10.1007/s11746-015-2629-0
Boskou D (2015) Olive and olive oil bioactive constituents. Academic Press and AOCS Press, Amsterdam.https://doi.org/10.1016/C2015-0-02391-7
Busquets R, Puignou L, Galceran MT, Skog K (2006) Effect of red wine marinades on the formation of heterocyclic amines in fried chicken breast. J Agric Food Chem 54:8376–8384. https://doi.org/10.1021/jf0616311
Castro-Alves VC, Cordenunsi BR (2015) Total soluble phenolic compounds quantification is not as simple as it seems. Food Anal Methods 8:873–884. https://doi.org/10.1007/s12161-014-9961-0
Cicco N, Lattanzio V (2011) The influence of initial carbonate concentration on the Folin-Ciocalteu micro-method for the determination of phenolics with low concentration in the presence of methanol: a comparative study of real-time monitored reactions. Am J Anal Chem 2:840. https://doi.org/10.4236/AJAC.2011.27096
Díez-Betriu A, Romero A, Ninot A, Tres A, Vichi S, Guardiola F (2022) Effect of freezing, fast-freezing by liquid nitrogen or refrigeration to preserve premium extra virgin olive oil during storage. Eur Food Res Technol 248:2651–2663. https://doi.org/10.1007/s00217-022-04078-9
Everette JD, Bryant QM, Green AM, Abbey YA, Wangila GW, Walker RB (2010) Thorough study of reactivity of various compound classes toward the Folin− Ciocalteu reagent. J Agric Food Chem 58:8139–8144. https://doi.org/10.1021/jf1005935
Fuentes E, Báez ME, Bravo M, Cid C, Labra F (2012) Determination of total phenolic content in olive oil samples by UV–visible spectrometry and multivariate calibration. Food Anal Methods 5:1311–1319. https://doi.org/10.1007/s12161-012-9379-5
Gargouri B, Ammar S, Zribi A, Mansour AB, Bouaziz M (2013) Effect of growing region on quality characteristics and phenolic compounds of chemlali extra-virgin olive oils. Acta Physiol Plant 35:2801–2812. https://doi.org/10.1007/s11738-013-1312-z
Gorinstein S, Martin-Belloso O, Katrich E, Lojek A, Číž M, Gligelmo-Miguel N, Haruenkit R, Park Y-S, Jung S-T, Trakhtenberg S (2003) Comparison of the contents of the main biochemical compounds and the antioxidant activity of some Spanish olive oils as determined by four different radical scavenging tests. J Nutr Biochem 14:154–159. https://doi.org/10.1016/S0955-2863(02)00278-4
Karaosmanoglu H, Soyer F, Ozen B, Tokatli F (2010) Antimicrobial and antioxidant activities of Turkish extra virgin olive oils. J Agric Food Chem 58:8238–8245. https://doi.org/10.1021/jf1012105
Kelebek H, Selli S, Kola O (2017) Quantitative determination of phenolic compounds using LC-DAD-ESI-MS/MS in cv. Ayvalik olive oils as affected by harvest time. J Food Meas Charact 11:226–235. https://doi.org/10.1007/s11694-016-9389-x
Lechhab T, Lechhab W, Cacciola F, Salmoun F (2022) Sets of internal and external factors influencing olive oil (Olea europaea L.) composition: a review. Eur Food Res Technol 248:1069–1088. https://doi.org/10.1007/s00217-021-03947-z
Lechhab T, Salmoun F, Lechhab W, El Majdoub YO, Russo M, Testa Camillo MR, Trovato E, Dugo P, Mondello L, Cacciola F (2021) Determination of bioactive compounds in extra virgin olive oils from 19 Moroccan areas using liquid chromatography coupled to mass spectrometry: a study over two successive years. Eur Food Res Technol 247(12):2993–3012. https://doi.org/10.1007/s00217-021-03842-7
Laincer F, Laribi R, Tamendjari A, Arrar L, Rovellini P, Venturini S (2014) Olive oils from Algeria: phenolic compounds, antioxidant and antibacterial activities. Grasas Aceites 65:e001–e001. https://doi.org/10.3989/gya.035713
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747. https://doi.org/10.1093/ajcn/79.5.727
Owen R, Giacosa A, Hull W, Haubner R, Spiegelhalder B, Bartsch H (2000) The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur J Cancer 36:1235–1247. https://doi.org/10.1016/S0959-8049(00)00103-9
Scatigno C, Festa G (2022) FTIR coupled with machine learning to unveil spectroscopic benchmarks in the Italian EVOO. Int J Food Sci Technol 57:4156–4162. https://doi.org/10.1111/ijfs.15735
Schendel RR (2019) Phenol content in sprouted grains. In: Feng H, Nemzer B, DeVries JW (eds.) Sprouted Grains. AACC International Press, Amsterdam, pp 247–315. https://doi.org/10.1016/B978-0-12-811525-1.00010-5
Shahbaz M, Sacanella E, Tahiri I, Casas R (2021) Mediterranean diet and role of olive oil, olives and olive oil in health and disease prevention. Academic Press, Amsterdam.https://doi.org/10.1016/C2018-0-05075-6
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158. https://doi.org/10.1038/nprot.2007.102
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol Academic Press, Amsterdam. https://doi.org/10.1016/S0076-6879(99)99017-1
Tahir HE, Xiaobo Z, Jianbo X. Mahunu GK, Jiyong S, Xu JL, Sun D-W (2019) Recent progress in rapid analyses of vitamins, phenolic, and volatile compounds in foods using vibrational spectroscopy combined with chemometrics: a review. Food Anal Methods 12:2361–2382.https://doi.org/10.1007/s12161-019-01573-w
Violino S, Taiti C, Marone E, Pallotino F, Costa C (2022) A statistical tool to determine the quality of extra virgin olive oil (EVOO). Eur Food Res Technol 248:2825–2832. https://doi.org/10.1007/s00217-022-04092-x
Visioli F, Poli A, Gall C (2002) Antioxidant and other biological activities of phenols from olives and olive oil. Med Res Rev 22:65–75. https://doi.org/10.1002/med.1028
Young I, Woodside J (2001) Antioxidants in health and disease. J Clin Pathol 54:176–186. https://doi.org/10.1136/jcp.54.3.176
Yubero-Serrano EM, Lopez-Moreno J, Gomez-Delgado F, Lopez-Miranda J (2019) Extra virgin olive oil: more than a healthy fat. Eur J Clin Nutr 72:8–17. https://doi.org/10.1038/s41430-018-0304-x
Acknowledgements
The authors would like to thank the Researchers Supporting Project number (RSP2023R138), King Saud University, Riyadh, Saudi Arabia.
Funding
The authors would like to thank the Researchers Supporting Project number (RSP2023R138), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
N.B.R., A.F., M.R.K., M.S.K, S.A, and R.B.: conceptualization and data curation; N.B.R, A.F., S.A., M.S.K.: formal analysis; M.R.K.: funding acquisition; N.B.R., A.F., M.S.K., S.A.: investigation; N.B.R., M.R.K.: methodology; N.B.R.: project administration; M.R.K, M.S.K.: resources; N.B.R., R.B., and M.R.K.: supervision; F.O.: validation; N.B.R., R.B., and M.R.K.: writing—original draft; N.B.R., R.B., and M.R.K.: writing—review and editing. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
Nayab Batool Rizvi that she has no conflict of interests. Anam Fatima that she has no conflict of interests. Rosa Busquets that she has no conflict of interests. Mohammad Rizwan Khan that he has no conflict of interests. Sadia Ashraf that she has no conflict of interests. Muhammad Shuaib Khan that he has no conflict of interests. Fatih Oz that he has no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rizvi, N.B., Fatima, A., Busquets, R. et al. Effect of the Media in the Folin-Ciocalteu Assay for the Analysis of the Total Phenolic Content of Olive Products. Food Anal. Methods 16, 1627–1634 (2023). https://doi.org/10.1007/s12161-023-02527-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-023-02527-z