Abstract
Proteins and amino acids are minor components of honey that are rarely used for its quality evaluation, although these components create its biological activity as well as can serve for overheating detection. The aim of the study was to use these indicators to confirm a declared on the label variety. Fifty-eight honey samples of 8 different varieties meeting the commercial quality requirements were used, including 28 local Polish and 30 commercial (mainly imported from EU and non-EU) honeys. For honey protein profiling previously used, polyacrylamide electrophoresis in denaturing conditions (SDS-PAGE) was applied whereas the free amino acid profile was analyzed by high-performance thin-layer chromatography (HPTLC) for the first time. As auxiliary indicators, the colorimetric determination of the protein content by the Bradford method, the activity of 5 glycolytic enzymes, including diastase, β-galactosidase, N-acetyl-β-glucosaminidase, α-mannosidase, and α-glucosidase, and the content of proline were used. It has been shown that based on the determined model protein SDS-PAGE profiles for selected monofloral honeys, it is possible to detect honeys of questionable variety based on the lack of specific protein bands or their diverging intensity. The HPTLC amino acid analysis can serve as a supporting control tool, capturing differences in the amino acid profile. Due to a great variation of multifloral honey, such assays are effective for monofloral honey only. The colorimetric assays, especially for total protein and β-galactosidase, can be also useful. The applied tools can be proposed for the initial verification of honey variety for cost reduction of officially recognized melissopalynological analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Honey is a popular food product, readily consumed for its taste, nutritional, and health benefits (Kumar and Bhowmik 2010; Rana et al. 2018). Good-quality honey can reach a high price on the market, but nevertheless, it is accepted by conscious consumers. Among the factors influencing the quality and properties of honey, three main groups can be distinguished: related to bees (species, health status, nutrition), geographical and climatic factors (including environmental pollution and floral origin), and factors related to the honey itself and its production (beehive technology, harvesting, maturation, decrystallization, handling, packaging, and storage) (Bett 2017; Mohammed 2020).
Depending on the main source used by bees, honey is categorized as nectar (mono- and multifloral) or honeydew, made from the secretions of trees and insects, mainly from the Aphidae family (Pita-Calvo and Vasquez 2017). Based on the botanical origin of the nectar, individual varieties of monofloral honeys are classified. While distinguishing nectar honey from honeydew honey is not difficult with the use of simple physicochemical analysis, the determination of the correct variety of nectar honey is more complicated and the only officially recognized method for determining the variety is the laborious and time-consuming melissopalynological analysis (von der Ohe et al. 2004; Thrasyvoulou et al. 2018). Alternative cost- and time-saving tools to verify the declared honey variety are still in demand. Multiple attempts are made to use chemical markers, phytochemicals mainly from the group of polyphenolic compounds (Gašić et al. 2017; Stanek and Jasicka-Misiak 2018; Beceril-Sanchez et al. 2021; Puścion-Jakubik et al. 2022), characteristic for a given variety or crop plants, and volatile compounds (Yang et al. 2018; Machado et al. 2020; Tananaki et al. 2022). Due to their dual origin (bee and plant), nitrogen compounds, mainly proteins, could be interesting but rarely studied group of honey components that can be used in quality assessment and variety verification (Baroni et al. 2002; Miłek et al. 2021; Muresan et al. 2022). Although there is little protein in honey (on average, from 0.3 g/100 g for nectar honeys to 0.6 mg/100 g for honeydew honey) (Bogdanov 2011), they are important ingredients, largely responsible for honey bioactivity. Glucose oxidase and active peptides, e.g., defensin-1, are recognized factors shaping antimicrobial activity (Kwakman and Zaat 2012; Bucekova et al. 2014). The main group of proteins is enzymes, such as diastase, invertase, glucose oxidase, acid phosphatase, catalase, and β-glucosidase, which due to their thermolability may be a good and sensitive indicator of honey quality (Machado de Melo et al. 2018; Miłek et al. 2021). Additionally, honey also contains free amino acids, among which proline usually predominates (Hermosin et al. 2003; Kowalski et al. 2017). Honey amino acid profile could be a potential indicator of honey variety (Balkanska et al. 2020; Pacholczyk-Sienicka et al. 2022).
Another aspect important for the classification of honey is its geographical origin. It has been shown that honeys purchased from local producers usually meet the requirements of the standards and show better health-promoting properties (Dżugan et al. 2018; Miłek et al. 2021). Often, commercially available honeys are blends from many countries, and in addition, inappropriate conditions are used in their production, i.e., too high temperature for liquefaction, which affects their parameters, including the content and activity of proteins.
One of the tools helpful in analyzing the protein profile of honey is polyacrylamide gel electrophoresis (PAGE). This technique was successfully used earlier to analyze the composition of honey protein fractions, to detect active enzymes (native-PAGE zymography), and the first attempts to use it to evaluate typical varietal patterns of protein bands were also made (Bilikova et al. 2015; Bocian et al. 2019; Borutinskaite et al. 2018; Miłek et al. 2021, Muresan et al. 2022). A similar assumption can be made for using the high-performance thin-layer chromatography (HPTLC) technique to create typical profiles of free amino acids in honey; however, to our knowledge, this technique has not yet been applied.
The aim of the study was to check the usefulness of honey protein and amino acid profiling to verify the variety declared on the label of both local and imported honeys. The study covered honey varieties typical for beekeeping in Central and Eastern Europe. Two not very common methods, SDS-PAGE and HPTLC, were tested as potential analytical tools in comparison to colorimetric standard measures of protein, diastase, and proline contents as well as selected glycolytic enzyme activity.
Material and Methods
Reagents and Chemicals
All used reagents were of analytical grade. The amino acid standards, p-nitrophenyl substrates for enzymatic activity assays, Nonidet P-40 Substitute, dithiotreitol, Coomassie Brilliant Blue G-250, and 2-methoxyethanol were purchased form Sigma-Aldrich (Saint Louis, MO, USA). Ninhydrin, acetic acid, isopropanol, n-propanol, and hydrochloric acid were purchased from Chempur (Gliwice, Poland). Deionized water was used to prepare the solutions.
Honey Samples
At the beginning, 80 honey samples (35 local, L; and 45 commercial, C) were initially assessed based on obligatory regulations (Journal of Laws 2015) in terms of honey quality and only 58 samples (28 L and 30 C) that fulfilled the law limits regarding water content, free acidity, conductivity, and HMF content (data not shown) were used in further study. Local honeys (L) were purchased directly from beekeepers in South-Eastern Poland (Podkarpackie region) whereas commercial honeys (C), mainly imported from EU and non-EU countries (according to labeling), were bought in local supermarkets (Table S1). The following varietal honeys were analyzed: rape—Brassica napus (4 L and 4 C); acacia—Robinia pseudoacacia (4 L and 4 C); linden—Tilia sp. (4 L and 4 C); buckwheat—Fagopyrum esculentum (4 L and 4 C); deciduous honeydew (3 L and 4 C); coniferous honeydew—mainly Abies sp. (3 L and 4 C); heather—Calluna vulgaris (2 L and 2 C); and multifloral (4 L and 4 C). The variety was declared by beekeeper for local and by producer/distributor for commercial honey. Until the analysis, honeys were stored in a dark place at a temperature of 20–22 °C.
Protein Content
In honey extracts (20% w/v), soluble protein fraction was determined by the Bradford method according to Latimer (2016). Accordingly, 1000 µL of Bradford reagent (G-250) was added to 100 μL of honey extract. Samples were incubated for 5 min at 20–22 °C and the absorbance (y) was read against blank sample at 595 nm using a spectrophotometer (Biomate 3, Thermo Scientific, Waltham, MA, USA). The results were calculated on the basis of a calibration curve 6.25–200 µg/per 20 µl of sample (x); (y = 31.6752x, r2 = 0.9919). Bovine albumin was used as a standard protein.
Proline Content
The proline content was determined in accordance with the instructions contained in the Polish regulation, relating to the methods of honey analysis (Journal of Laws 2015). Honey solutions at a concentration of 2.5 g in 50 mL of distilled water were prepared. Five hundred microliters of each honey solution, 0.25 mL of concentrated formic acid, and 1 mL of a 3% solution of ninhydrin in 2-methoxyethanol were added to the test tube. The closed samples were heated at 100 °C for 15 min. After this time, the samples were cooled until ambient temperature is reached, and 5 mL of 50% isopropanol was added. The absorbance at 520 nm was measured (y) against the blank prepared for each honey (0.5 mL honey solution, 1.25 mL distilled water, 5 mL isopropanol). The results were calculated on the basis of calibration curve prepared for proline in the concentration range 0–20 µg/per 500 µl of sample (x); (y = 0.0259x, r2 = 0.9655).
Enzyme Activity
Diastase activity in honey was determined using Phadebas Honey Diastase Test tablets (Magle AB, Sweden) by the spectrophotometric method by following the instructions provided by the manufacturer. The absorbance at 620 nm was measured against the control. The results were converted according to the formula included in the instruction and expressed as diastase number (DN).
The activities of four glycosidases, N-acetyl-β-glucosaminidase (NAG), α-mannosidase (α-MAN), β-galactosidase (β-GAL), and α-glucosidase (α-GLU), using the method described by Tomczyk et al. (2019) were tested for honey samples. The reaction mixture contained 25 μL of 2 mM appropriate p-nitrophenyl substrate in 0.2 M citrate buffer (pH 5.5 for α-GLU and 4.0 for other enzymes) and 25 μL of honey aqueous solution (20% w/w) sample. In blank, 25 μL of suitable buffer was used instead of substrate solution. Samples were incubated at 37 °C for 60 min, and the reaction was then stopped by the addition of 250 μL of 0.5 M carbonate buffer at pH 10.5. Absorbance was measured on a microplate reader (EPOCH2, Biotek) at a wavelength of 400 nm. Results were expressed as enzymatic units mU (nmol/min/g) by multiplying the measured absorbance by a factor of 79.4.
Protein Profiling by SDS-PAGE
Samples of each honey (0.5 g) were prepared for denaturing electrophoresis as described previously (Tomczyk et al. 2022) by adding 0.5 mL of deionized water containing 2% Nonidet P-40 Substitute, and 2% dithiothreitol and mixed thoroughly on a rotator. Liquid honey samples were combined with 4 × concentrated Laemmli buffer (20 µL sample + 10 µL buffer). Samples were incubated for 5 min at 95 °C and then cooled before being applied to 15% denaturing gels (with 3% stacking gels). The samples (15 µL) were loaded onto 8 different gels, each of which contained samples of one species of honey. Electrophoresis was carried out on a Mini-Protean Tetra Cell apparatus (Bio-Rad Laboratories, Hercules, CA, USA) at 50 V for 15 min and then at 200 V for 1 h according to the standard method of Laemmli, with the BlueEasy Prestained Protein Marker (NIPPON Genetics EUROPE, Düren, Germany) as a molecular weight marker and Tris–Glycine-SDS buffer. After electrophoresis, gels were stained overnight with Coomassie Brilliant Blue G-250 and destained for 24 h with deionized water. Gels were scanned with Image Scanner III (GE Healthcare, Little Chalfont, UK) and processed by EPSON Scan software. Gels analysis was performed in ImageJ (1.52a) software to generate a graphical representation of each lane on the gel to assist in sample comparison. For better visualization, individual bands from the SDS-PAGE gel were contrasted with the profile obtained with the software.
HPTLC Free Amino Acid Profiling
Free amino acid profiles of all honey samples were obtained using CAMAG HPTLC system (automatic applicator Linomat 5, an automatic developing chamber ADC2, a derivatizer, and a visualization apparatus TLC Visualizer 2, CAMAG, Switzerland). Chromatographic separation was performed on silica gel 60 F254 HPTLC glass plates (20 × 10 cm), purchased from Merck (Darmstadt, Germany). The mobile phase was a mixture of n-propanol and water (7:3, v/v), according to Brunauer et al. (2014). Honey solutions (20% in water) were applied to the plate in a volume of 4 µL as bands 6 mm wide, 8 mm from the bottom edge of the plate. Standards of 21 protein amino acids were used and applied in a volume of 2 µL (1 mg/mL in distilled water). The plates were developed with a ninhydrin solution (0.2 g in 100 ml of 96% ethanol, acidified with 3 mL of acetic acid) and after spraying were heated at 105 °C for 3 min. The plates were photographed under visible light and analyzed with the Vision CATS software (CAMAG). Based on a comparison of the colors of the bands and their retention factor (Rf) values, specific amino acids for each honey varieties were identified.
Statistical Analysis
All analyses of quantitative parameters were performed in triplicates; data are shown as mean ± standard deviation. The correlations among obtained parameters were analyzed by Pearson coefficient (r). The significant differences were calculated by a one-way analysis of variance, followed by Tukey’s honest significant difference test (p < 0.05) or t-test in the case of comparison of local and imported honey samples. Principal components analysis (PCA) was applied to find the relation between tested parameters. All calculations were made using the Statistica 13.1 software (StatSoft, Tulsa, OK, USA).
Results and Discussion
Protein and Proline Content
The analysis of protein content (Table 1) using the Bradford method showed that dark honeys, especially heather honeys, contain the highest amount of protein, which is in line with previous studies (Burzyńska et al. 2020; Miłek et al. 2021). However, the protein content of heather honeys varied depending on the geographical origin of the honey. Significantly lower values (by 65.48%) were found for imported heather honeys which were also more diversified. Lower values were obtained for buckwheat and honeydew honeys (from 90.19 to 177 mg/100 g depending on the variety). The lowest values among the analyzed samples were found for light honeys (rape, acacia, and linden). In this case, the average protein content ranged between 34.36 and 66.69 mg/100 g depending on the variety. When comparing local with imported honeys, it should be noted that local honeys are richer in protein, especially dark honeys (buckwheat and deciduous honeydew). For linden and coniferous honeydew honey, slightly higher protein content in commercial honeys was observed (from 2.29 to 7.78%); p > 0.05. Only commercial rape honeys had a much higher protein content than local honeys (30.02%); p < 0.05.
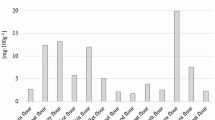
A similar relationship was found when analyzing the proline content in varietal honeys (Fig. 1). It was shown that light honeys, mainly rape and acacia honeys, showed the lowest values of proline content (on average 33.79 mg/100 g). Much higher values were found for dark honeys (57.35–122.36 mg/100 g), and the highest values were obtained for heather honeys (110.51–140.12 mg/100 g). Truzzi et al. (2014) analyzed the proline content in Italian varietal honeys. The authors showed that the honeydew honeys contained 66.6 mg of proline in 100 g of honey, which confirms the results presented in this study. In turn, the proline contents of 25.3 mg/100 g and 39.7 mg/100 g were found for acacia and linden honey, respectively. The extended analysis was performed by Czipa et al. (2012) who showed that Hungarian acacia honeys contain from 20.7 to 44.7 mg of proline per 100 g of honey, which is consistent with the results shown in Fig. 1. Moreover, for acacia honeys originating from outside the EU, they showed on average a higher proline content. The linden honeys from the European Union countries analyzed by the authors contained on average 14% less proline compared to Polish honeys. The authors also studied less known honeys such as coriander honey, in which they found even 228.83 mg of proline in 100 g of the product. Because the proline content is considered an indicator of honey maturity and its possible adulteration, it is assumed that honey should contain at least 18 mg/100 g of this amino acid (Bogdanov et al. 1999). Thus, the tested samples meet this assumption well.
Enzymatic Activity
Diastase activity is the only enzymatic parameter which is limited by law regulations. The comparison of enzymatic activity in varietal honeys depending on the origin of the sample is presented in Table 1.
The analyzed honeys showed diversified diastase activity depending on the variety, which was expressed as the variation coefficient (10–30%). Again, the highest values of up to 44.21 DN were found for dark honeys (honeydew from coniferous and deciduous honeydew and heather), while the lowest values (10.73 DN) were found for light honeys (acacia, rape, linden). Moreover, it has been shown that honeys from local apiaries show higher values (up to 57.66% for honeydew honeys) compared to honeys from trade. The results presented in Table 1 are similar to those presented by Wesołowska and Dżugan (2017), who tested Polish varietal honeys. The authors showed that among the tested samples, honeydew honeys showed the highest diastase activity (33.08 DN), and rape honeys were the lowest (15.35 DN). However, Nayik and Nanda (2015) showed a higher diastase activity in acacia honeys (15.51 DN) than in multifloral honeys (14.93 DN).
Changes in the activity of other glycolytic enzymes in honey depending on the variety and origin were analyzed (Table 1). It was found that heather and honeydew honeys had a much higher N-acetyl-β-glucosidase (NAG) activity (up to 43.70 mU) compared to other types of honey (from 4.29 to 10.46 mU). Heather and honeydew honeys also showed an increased content of α-mannosidase and β-galactosidase. The higher β-galactosidase activity was also observed in rape honey (32.15 mU), which is much greater compared to other glycosidases. Thus, this enzyme could be a potential marker in confirming the authenticity of rape, heather, and honeydew honeys.
Unlike diastase which is included in the requirements for the commercial quality of honey, other enzymes belonging to glycosidases group are not widely studied (Bogdanov 2016; Dżugan et al. 2021; Sidor et. al. 2021). In other study, this enzyme was proposed as an indicator of honey thermal processing (Dżugan et al. 2021; Sidor et al. 2021). The studies showed that in all of the tested honeys, the glycosidase activity decreases in sequence: β-GAL > α-GLU > α-MAN and the highest activities for linden than multifloral and acacia honeys were found (Sidor et al. 2021). The results cited are in line with the present study. Moreover, reduced activity of the tested enzymes was found in fresh liquid honeys subjected to fixation and liquefied solid honey regardless of the technique used. The significant decrease found for glucosidase activity, especially β-galactosidase, as a result of honey processing by heat or ultrasound treatment indicated that the tested glycosidases are more sensitive to overheating than diastase (Dżugan et al. 2021). However, it should be remembered that enzymatic activity may change under the influence of various factors, depending on the degree of honey maturity and the method of processing (Karabournioti and Zervalaki 2001; Zhang et al. 2021).
As many studies have proven that chemometric approach based on multivariate statistical analysis (PCA or LDA) can be used for honey classification in terms of botanical and geographical origin as well as to detect adulteration in honey (Oroian and Ropciuc 2017; Kaczmarek et al. 2019; Miłek et al. 2021; Tarapatskyy et al. 2021). Following the study of Tarapatskyy et al. (2021) where a clear clustering for 4 varieties of Polish honey using principal component analysis (PCA) has been observed, we use the same approach to check the relation between determined parameters (Fig. 2). The results indicated the strong positive correlation between protein content and tested enzymatic activities, excluding α-glucosidase, demonstrated by close proximity on the biplot (Fig. 2a). Similarly, weak correlation was observed for protein and free proline content (Fig. 2a). It can be observed that almost all tested parameters located close to the projection circle have a significant impact on the quality of honey. However, among examined parameters, the most significant impact on overall classification had protein and proline content as well as α-GLU and NAG activity, based on their location close to the projection circle.
Among the parameters studied, the activity of N-acetylglucosaminidase strongly correlates with the total protein content (r = 0.839), as well as the activity of β-galactosidase relatively well (r = 0.614). Moreover, the values for the protein correlated well with the proline content (r = 0.524), which correlated to a similar degree with NAG (r = 0.533). The PCA score plot (Fig. 2b) does not distinguish separate honey groups. However, samples with high values of tested parameters are located on the right part of the graph (positive PC1 and PC2 values for heather and positive PC1 and negative PC2 values for honeydew honey). Samples of pale honeys are grouped in the left part (negative PC1 values) of the graph but there is no clear varietal clustering. Similarly, Tarapatskyy et al. (2021) observed a close resemblance between multifloral and linden honey samples whereas buckwheat and honeydew honey formed clearly separated clusters. However, they considered a different set of variables, including detailed polyphenol, sugar, and elemental composition as well as physicochemical parameters.
SDS-PAGE Protein Profiling
For the analysis of protein profiles using the SDS-PAGE technique, each variety was analyzed on a separate gel comparing local and commercial honeys. The gels resulted from electrophoretic separation were stained with Coomassie Brilliant Blue dye, and band patterns corresponding to individual proteins were obtained for each sample. As the honey solutions were applied to the gel at the same concentration, a direct comparison of the protein concentration in each honey due to the different intensity of staining bands after separation can be made. By processing in ImageJ software, protein profiles were obtained in the form of graphs, in which individual proteins are visualized in the form of peaks (Fig. 3).
Images of gels obtained for honey varieties. a Rape (R). b Acacia (A). c Linden (L). d Buckwheat (B). e Heather (HR). f Coniferous honeydew (CHD). M, protein mass marker (kDa). Standard profile, representative protein profiles considered as standard; questionable profile, example of a sample profile suspected of being adulterated or of lower quality
Among the analyzed varieties, rape, linden, and buckwheat honeys were characterized by the greatest homogeneity of profiles between individual samples. Only 12.5% of rape (1 sample of 8), 12.5% linden, 12.5% acacia, 14% coniferous honeydew, 25% buckwheat (2 samples of 8), and 25% heather honey samples were found to be inconsistent with the varietal standard. Rape honey tracks are dominated by two protein bands (masses of about 59 and 70 kDa); the signal from a protein of about 95 kDa is also clearly marked. Only for one sample (R5, local honey) a different profile from the others was obtained; only two peaks from proteins with masses of about 55 and 65 kDa were visible, which can contest the variety declared by the beekeeper. Moreover, in sample R10 (commercial blend), a low-weight protein fraction is visible at the lower edge of the gel. A typical profile, although different in terms of color intensity, was observed for acacia honey samples (Fig. 3b). It is dominated by two proteins, similar to rape honey (Fig. 3a). Two local samples (A1 and A3) had higher protein content (more intensive bands). The linden honeys have a typical stripe pattern, similar to rape, with slight differences in the intensity of the stripe corresponding to the protein with a mass of approx. 95 kDa visible for samples L2, L9, and L10 (Fig. 3c). In turn, buckwheat honey profiles are very characteristic for this variety, with an additional protein fraction in the range of 25–30 kDa, manifested by a wide peak with two maxima (Fig. 3d). In the upper part of the gels, a larger number of bands can be also clearly distinguished (in the range of 60–90 kDa). Two of the tested commercial honeys (B7 and B8) show much less intensely colored stripes, although still with the arrangement characteristic for buckwheat honey. Such profile differences may indicate worse quality of these honeys, either due to the wrong variety specification or overheating of the honey. This can only be verified by pollen analysis. Among the studied varieties of nectar honeys, heather honeys have the highest protein content (Table 1) and have a characteristic protein profile (Fig. 3e). It is dominated by three proteins with high masses (approx. 70 kDa, approx. 60 kDa, and approx. 48 kDa) as well as a very intense protein band with a mass of approx. 28 kDa. Among the analyzed samples, one (HR3—imported honey) does not show a typical profile, so it can be considered as a sample with an incorrectly defined variety. In the case of coniferous honeydew honeys, the profiles are more even; sample CHD4 (commercial) clearly stands out from the others, having additional peaks from proteins with masses of approx. 33 and 39 kDa and visible 2 peaks less intense than proteins and lower masses (Fig. 3f).
Similar protein profiles by SDS-PAGE have been obtained previously in other studies. Muresan et al. (2022) studied honeys of various geographic and botanical origins. The protein profiles obtained by them showed great similarity to those shown in this work; most of the main proteins visible on the gels were those with masses in the range of 45 to 85 kDa. They are considered to be proteins of bee origin (major royal jelly proteins, enzymes). The most intense bands were obtained in each case for a protein with a mass of about 57 kDa which they classified as MRJPs (50–60 kDa) (Lewkowski et al. 2019, Muresan et al. 2022). Alpha-glucosidase and MRJP-1 have been also identified as the main proteins of bee origin found in honey by Bocian et al. (2019). The low mass bands on the gels (up to several kDa) may be derived from peptides present in honey, e.g., royalisin and apisimin (Bilikova et al. 2015). Other proteins visible in the profiles may come from plants and constitute specific markers of botanical origin. Additional proteins with lower masses (in the range of 20–30 kDa) were obtained for honeys defined based on the melissopalynological analysis as buckwheat, sunflower, chestnut, or coriander (Muresan et al. 2022). Royal jelly proteins with a mass of 48 and 55 kDa (apalbumins) have been detected in honeys of various origins and are considered potential markers of honey quality and authenticity (Bilikova et al. 2015). Protein profiling by SDS-PAGE was also used as a tool to determine the quality of coniferous honeydew honeys. It has been shown that a high-mass protein (245 kDa) is characteristic of such honeys (Tomczyk et al. 2022). The comparison of honeydew honeys with other varieties made it possible to show nectar admixtures to some samples. As markers, proteins with lower masses (25–55 kDa), most probably derived from flower nectar (Tomczyk et al. 2022), were indicated. In the present study, the analysis was carried out on smaller gels; hence, it is impossible to demonstrate the presence or absence of the band at 245 kDa in the case of honeydew honeys. This indicates that more subtle differences between the varieties could be made visible by the use of high-resolution gels.
Proteomic analysis of varietal honeys in conjunction with the analysis of proteins derived from the pollen of various plants may also be a promising tool for the verification of the variety of honey (Baroni et al. 2002). Plant-based proteins and peptides may be promising markers of a botanical variety of honey, as demonstrated by the example of Manuka honey, in which 17 proteins derived from Leptospermum scoparium nectar were detected (Bong et al. 2021). The characteristic additional protein band was also found to be a varietal marker for Ziziphus jujuba honeys useful in the authentication of samples of this valuable honey (Zhang et al. 2019).
A high degree of variability was observed in the case of the analyzed deciduous honeydew honey (Fig. 4). This is probably related to the diversity of the source of the honey harvest, which is the honeydew, and to possible nectar admixtures in such honeys. In addition to typical proteins found in other varieties, additional peaks can be seen in the profiles of local honeydew honeys, derived from proteins with a mass of approx. 25–35 kDa (samples DHD1 and DHD3; a wide band with 3 visible maxima), or approx. 40 kDa (additional peak in sample DHD2). Commercial samples showed less variation. In the case of coniferous honeydew honeys, the profiles are more even; sample CHD4 clearly stands out from the others, having additional peaks from proteins with masses of approx. 33 and 39 kDa and visible 2 peaks less intense than proteins and lower masses.
As can be expected, the greatest diversity in the profiles of multifloral honeys was observed (Fig. 5). The sample M3 has additional proteins, similar to buckwheat honey. Samples of commercial multifloral honeys are low in proteins, which confirm the lower quality of these types of blends.
Previously, apart from the SDS-PAGE, also the native-PAGE technique was used to evaluate the quality of local honeys compared to imported blends. It has been shown that commercially available honeys are often characterized by lower protein content (Miłek et al. 2021). Using the native electrophoresis technique, different band patterns were obtained than in SDS-PAGE, which makes it impossible to directly compare the results with those presented in this study. It has been shown, however, that the zymography technique may be useful, e.g., by showing differences in the occurrence of amylase isoforms in honeys of various origins (Miłek et al. 2021). Zymography has also been used to characterize buckwheat honey. An in-depth proteomic analysis allowed for the identification of plant proteins (mainly enzymes from the hydrolases, lyases, and oxidoreductase classes) in addition to the main bee proteins of origin (from the MRJP family) (Borutinskaite et al. 2018).
Amino Acid Profile
The amino acid profiles were obtained for each variety on a separate thin-layer plate. Usually, profiling is performed by the HPLC method; for the first time, we used the HPTLC technique, which is cheaper and allows for simultaneous analysis of up to 20 samples on one chromatographic plate. Exemplary images of ninhydrin-derivatized HPTLC plates for the analyzed honey varieties are presented in Fig. 6.
All tested samples showed the presence of proline, visible as a yellow band at Rf = 0.23–0.26 (Fig. 6). Its concentration varied, but no clear relationship was found between the amount of proline and the botanical origin of honey. The most intense violet band present in all samples at Rf = 0.41 belongs to an unidentified compound, most probably not belonging to protein amino acids. For buckwheat honey, the characteristic amino acid is isoleucine (red band at Rf = 0.50), which can be considered a marker of this variety (Fig. 6b). It is also present in other honey varieties, but in a much lower concentration. Literature data confirm the exceptionally high content of isoleucine and leucine in Polish buckwheat honeys, compared to other varieties (Janiszewska et al. 2012). In samples of honeydew honeys, especially from deciduous honeydew (Fig. 5d), there is a distinct band assigned to aspartic acid (red, Rf = 0.28).
Generated profiles for tested honey varieties are presented in Fig. 7 which can be used as the standard for comparison with analyzed samples. The observed differences mainly concerned the lower activity of proline which is known as a marker of good honey quality (R3, A2, L10 samples). However, in some cases, different image was observed, suggesting the unclear variety declaration in questionable samples (B8: lack of isoleucine, CHD6; DHD8: lack of aspartic acids; or HR3: unexpected isoleucine and aspartic acid).
As in the case of protein profiles, also the amino acid profiles of multifloral honeys were very diversified. Additional amino acids appeared in them, such as leucine and tyrosine as well as aspartic acid (Fig. 8).
We propose the use of a relatively simple tool, which is thin-layer chromatography for the initial selection of honeys of adulterated variety or as a support for verifying honey variety by melissopalynology. Among tested honeys, 12.5% of acacia, 25% of buckwheat and heather, and 37.5% of linden honey were pointed out as questionable based on the analysis of amino acid profiles using the HPTLC method. Samples classified as not inconsistent with the reference profiles require variety verification by pollen analysis. The exemplary profiles of the lower quality samples shown in Fig. 6 were characterized either by negligible intensity of the amino acid bands or additional bands from the unusual amino acids of the given variety. In the case of a sample of heather honey (HR3), HPTLC analysis confirms the earlier hypothesis that it is honey with an incorrectly defined variety. It is distinguished by the presence of an intense band probably derived from isoleucine, which may suggest a high proportion of buckwheat nectar.
Amino acid profile analysis has previously been used as a tool to support the identification of the botanical origin of honey as well as the diversification of geographic origin (Cometto et al. 2003; Hermosin et al. 2003; Sun et al. 2017; Kowalski et al. 2017). Using chromatographic analysis supported by chemometric analysis, it was also shown that the content of individual free amino acids in honey and the ratio of exo- to endogenous amino acids vary depending on the origin of the honey. Such data may be useful in confirming the authenticity of honey as an extension of standard methods of quality evaluation and melissopalynological analysis limitation (Łozowicka et al. 2021).
Conclusions
The research showed the possibility of using the SDS-PAGE protein profile and the amino acid HPTLC to verify the beekeeper’s or producer’s declaration regarding the variety of Polish local and imported honey samples. The proposed indicators make it possible to confirm or question the declared variety and refer questionable samples to melissopalynological analysis. As an aid, a simple Bradford protein determination and the β-galactosidase activity can be used as a valuable marker, especially for heather, rape, and honeydew honeys. The proposed analytical tools can allow for the initial screening of honeys available on the market in terms of the adulteration of variety declaration which can limit the cost of pollen analysis in beekeeping. However, the implementation of methods in beekeeping practice requires verification for a larger number of honey samples. Moreover, the effectiveness of the proposed methods for honey varieties other than those produced in Central and Eastern Europe requires separate studies. The validation of the usefulness of proteins and amino acids as markers of honey authenticity would provide a simple and affordable tools that beekeeping practice is waiting for.
Data Availability
The data presented in this study are available in the article and supplementary material.
References
Balkanska R, Stefanova K, Stoikova-Grigorova R (2020) Main honey botanical components and techniques for identification: a review. J Apic Res 59:852–861. https://doi.org/10.1080/00218839.2020.1765481
Baroni MV, Chiabrando GA, Costa C, Wunderlin DA (2002) Assessment of the floral origin of honey by SDS-page immunoblot techniques. J Agric Food Chem 50:1362–1367. https://doi.org/10.1021/jf011214i
Becerril-Sánchez AL, Quintero-Salazar B, Dublán-García O, Escalona-Buendía HB (2021) Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants 10:1–23. https://doi.org/10.3390/antiox10111700
Bett CK (2017) Factors influencing quality honey production. Int J Acad Res Bus Soc Sci 7:281–292. https://doi.org/10.6007/ijarbss/v7-i11/3458
Bilikova K, Krakova TK, Yamaguchi K, Yamaguchi Y (2015) Major royal jelly proteins as markers of authenticity and quality of honey. Arh Hig Rada Toksikol 66:259–267. https://doi.org/10.1515/aiht-2015-66-2653
Bocian A, Buczkowicz J, Jaromin M et al (2019) An effective method of isolating honey proteins. Molecules 24:10–12. https://doi.org/10.3390/molecules24132399
Bogdanov S (2011) Physical properties of honey: honey composition. The honey book; University of Belgrade, Belgrade, Serbia
Bogdanov S, Lüllmann C, Martin P et al (1999) Honey quality and international regulatory standards: review by the international honey commission. Bee World 80:61–69. https://doi.org/10.1080/0005772x.1999.11099428
Bogdanov S (2016) Honey technology. In: The book of honey. Bee Product Science. http://www.bee-hexagon.net/. Accessed 10 July 2022
Bong J, Middleditch M, Loomes KM, Stephens JM (2021) Proteomic analysis of honey. Identification of unique peptide markers for authentication of NZ mānuka (Leptospermum scoparium) honey. Food Chem 350:128442. https://doi.org/10.1016/j.foodchem.2020.128442
Borutinskaite V, Treigyte G, Čeksteryte V et al (2018) Proteomic identification and enzymatic activity of buckwheat (Fagopyrum esculentum) honey based on different assays. J Food Nutr Res 57:57–69
Brunauer LS, Caslavka KE, Van Groningen K (2014) Ion exchange and thin layer chromatographic separation and identification of amino acids in a mixture: an experiment for general chemistry and biotechnology laboratories. J Chem Educ 91:2216–2220. https://doi.org/10.1021/ed500226q
Bucekova M, Valachova I, Kohutova L et al (2014) Honeybee glucose oxidase - its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys. Naturwissenschaften 101:661–670. https://doi.org/10.1007/s00114-014-1205-z
Burzyńska M, Piasecka-Kwiatkowska D, Springer E (2020) Allergenic properties of Polish nectar honeys. Acta Sci Pol Technol Aliment 19:15–24. https://doi.org/10.17306/j.afs.2020.0750
Cometto PM, Faye PF, Di Paola Naranjo RD et al (2003) Comparison of free amino acids profile in honey from three Argentinian regions. J Agric Food Chem 51:5079–5087. https://doi.org/10.1021/jf021081g
Czipa N, Borbély M, Gyori Z (2012) Proline content of different honey types. Acta Aliment 41:26–32. https://doi.org/10.1556/AAlim.2011.0002
Dżugan M, Ruszel A, Tomczyk M (2018) Quality of imported honeys obtainable on the market in the podkarpacie region. Zywn Nauk Technol Jakosc/Food Sci Technol Qual 25:127–139
Dżugan M, Grabek-Lejko D, Sidor E, Tomczyk M (2021) The impact of ultrasound decrystallization on enzymatic, antioxidant and antibacterial properties of honey. Innov Food Sci Emerg Technol 71:102709. https://doi.org/10.1016/j.ifset.2021.102709
Gašić UM, Milojković-Opsenica DM, Tešić ŽL (2017) Polyphenols as possible markers of botanical origin of honey. J AOAC Int 100:852–861. https://doi.org/10.5740/jaoacint.17-0144
Hermosín I, Chicón RM, Cabezudo MD (2003) Free amino acid composition and botanical origin of honey. Food Chem 83:263–268. https://doi.org/10.1016/S0308-8146(03)00089-X
Janiszewska K, Aniołowska M, Nowakowski P (2012) Free amino acids content of honeys from Poland. Polish J Food Nutr Sci 62:85–89. https://doi.org/10.2478/v10222-011-0041-5
Journal of Laws of the Republic of Poland (2015) Regulation of the Minister of Agriculture and Rural Development of Poland of 30 July 2015 changing the regulation on analysis methods related to the evaluation of honey (Dz.U. 2015 poz. 1173) Available on: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150001173
Kaczmarek A, Muzolf-Panek M, Tomaszewska-Gras J, Konieczny P (2019) Predicting the botanical origin of honeys with chemometric analysis according to their antioxidant and physicochemical properties. Polish J Food Nutr Sci 69:191–201. https://doi.org/10.31883/pjfns/108526
Karabournioti S, Zervalaki P (2001) The effect of heating on honey HMF and invertase. Apiacta 36:177–181
Kowalski S, Kopuncová M, Ciesarová Z, Kukurová K (2017) Free amino acids profile of Polish and Slovak honeys based on LC–MS/MS method without the prior derivatisation. J Food Sci Technol 54:3716–3723. https://doi.org/10.1007/s13197-017-2838-7
Kumar KS, Bhowmik D (2010) Medicinal uses and health benefits of honey: an overview. J Chem Pharm Res 2:385–395
Kwakman PHS, Zaat SAJ (2012) Antibacterial components of honey. IUBMB Life 64:48–55. https://doi.org/10.1002/iub.578
Latimer GW (2016) Official methods of analysis of AOAC International - 20th Edition; AOAC International: Gaithersburg, MD, USA. Available on: https://www.techstreet.com/standards/official-methods-of-analysis-of-aoac-international-20th-edition-2016?product_id=1937367
Lewkowski O, Mureșan CI, Dobritzsch D et al (2019) The effect of diet on the composition and stability of proteins secreted by honey bees in honey. Insects 10:282. https://doi.org/10.3390/insects10090282
Łozowicka B, Kaczyński P, Iwaniuk P (2021) Analysis of 22 free amino acids in honey from Eastern Europe and Central Asia using LC-MS/MS technique without derivatization step. J Food Compos Anal 98:103837. https://doi.org/10.1016/j.jfca.2021.103837
Machado AM, Miguel MG, Vilas-Boas M, Figueiredo AC (2020) Honey volatiles as a fingerprint for botanical origin — a review on their occurrence on monofloral honeys. Molecules 25:374. https://doi.org/10.3390/molecules25020374
Machado De-Melo AA, de Almeida-Muradian LB, Sancho MT, Pascual-Maté A (2018) Composition and properties of Apis mellifera honey: a review. J Apic Res 57:5–37. https://doi.org/10.1080/00218839.2017.1338444
Miłek M, Bocian A, Kleczyńska E et al (2021) The comparison of physicochemical parameters, antioxidant activity and proteins for the raw local polish honeys and imported honey blends. Molecules 26:2423. https://doi.org/10.3390/molecules26092423
Mohammed MEA (2020) Factors affecting the physicochemical properties and chemical composition of bee’s honey. Food Rev Int 38:1330–1341. https://doi.org/10.1080/87559129.2020.1810701
Mureșan CI, Cornea-Cipcigan M, Suharoschi R et al (2022) Honey botanical origin and honey-specific protein pattern: characterization of some European honeys. Lwt 154:112883. https://doi.org/10.1016/j.lwt.2021.112883
Nayik GA, Nanda V (2015) Physico-chemical, enzymatic, mineral and colour characterization of three different varieties of honeys from Kashmir valley of India with a multivariate approach. Polish J Food Nutr Sci 65:101–108. https://doi.org/10.1515/pjfns-2015-0022
Oroian M, Ropciuc S (2017) Honey authentication based on physicochemical parameters and phenolic compounds. Comput Electron Agric 138:148–156. https://doi.org/10.1016/j.compag.2017.04.020
Pacholczyk-Sienicka B, Ciepielowski G, Modranka J et al (2022) Classification of Polish natural bee honeys based on their chemical composition. Molecules 27:4844. https://doi.org/10.3390/molecules27154844
Pita-Calvo C, Vázquez M (2017) Differences between honeydew and blossom honeys: a review. Trends Food Sci Technol 59:79–87. https://doi.org/10.1016/j.tifs.2016.11.015
Puścion-Jakubik A, Karpińska E, Moskwa J, Socha K (2022) Content of phenolic acids as a marker of polish honey varieties and relationship with selected honey-quality-influencing variables. Antioxidants 11:1312. https://doi.org/10.3390/antiox11071312
Rana S, Mishra M, Yadav D et al (2018) Medicinal uses of honey: a review on its benefits to human health. Prog Nutr 20:5–14. https://doi.org/10.23751/pn.v20i1-S.6394
Sidor E, Tomczyk M, Dżugan M (2021) Application of ultrasonic or microwave radiation to delay crystallization and liquefy solid honey. J Apic Sci 65:243–253. https://doi.org/10.2478/jas-2021-0027
Stanek N, Jasicka-Misiak I (2018) HPTLC phenolic profiles as useful tools for the authentication of honey. Food Anal Methods 11:2979–2989. https://doi.org/10.1007/s12161-018-1281-3
Sun Z, Zhao L, Cheng N et al (2017) Identification of botanical origin of Chinese unifloral honeys by free amino acid profiles and chemometric methods. J Pharm Anal 7:317–323. https://doi.org/10.1016/j.jpha.2017.06.009
Tananaki C, Liolios V, Kanelis D, Rodopoulou MA (2022) Investigation of volatile compounds in combination with multivariate analysis for the characterization of monofloral honeys. Appl Sci 12:264. https://doi.org/10.3390/app12010264
Tarapatskyy M, Sowa P, Zaguła G et al (2021) Assessment of the botanical origin of polish honeys based on physicochemical properties and bioactive components with chemometric analysis. Molecules 26:4801. https://doi.org/10.3390/molecules26164801
Thrasyvoulou A, Tananaki C, Goras G et al (2018) Legislation of honey criteria and standards. J Apic Res 57:88–96. https://doi.org/10.1080/00218839.2017.1411181
Tomczyk M, Miłek M, Sidor E, Kapusta I, Litwińczuk W, Puchalski C, Dżugan M (2019) The effect of adding the leaves and fruits of morus alba to rape honey on its antioxidant properties, polyphenolic profile, and amylase activity. Molecules 25(1):84. https://doi.org/10.3390/molecules25010084
Tomczyk M, Bocian A, Sidor E et al (2022) The use of HPTLC and SDS-PAGE methods for coniferous honeydew honey fingerprinting compiled with mineral content and antioxidant activity. Molecules 27:720. https://doi.org/10.3390/molecules27030720
Truzzi C, Illuminati S, Annibaldi A et al (2014) Physicochemical properties of honey from marche, central Italy: classification of unifloral and multifloral honeys by multivariate analysis. Nat Prod Commun 9:1595–1602. https://doi.org/10.1177/1934578x1400901117
Von der Ohe W, Oddo L, Piana M et al (2004) Harmonized methods of melissopalynology. Apidologie 35:S18–S25. https://doi.org/10.1051/apido:2004050
Wesołowska M, Dżugan M (2017) Activity and thermal stability of diastase present in honey from Podkarpacie region. Zywn Nauka Technol Jakosc/Food Sci Technol Qual 24:103–112. https://doi.org/10.15193/zntj/2017/113/214
Yang Y, Battesti MJ, Costa J et al (2018) Volatile components as chemical markers of the botanical origin of Corsican honeys. Flavour Fragr J 33:52–62. https://doi.org/10.1002/ffj.3414
Zhang Y, Wang Y, Zhao H et al (2019) Characterization of novel protein component as marker for floral origin of jujube (Ziziphus jujuba Mill.) honey. J Agric Food Chem 67:12255–12263. https://doi.org/10.1021/acs.jafc.9b05190
Zhang GZ, Tian J, Zhang YZ et al (2021) Investigation of the maturity evaluation indicator of honey in natural ripening process: the case of rape honey. Foods 10:2882. https://doi.org/10.3390/foods10112882
Funding
This work was supported by the project financed under the project “Nitrogen compounds as a new tool to verify the variety and authenticate honey origin” (No. 702/2021) by The National Support Centre for Agriculture within the mechanism of the Common Agricultural Policy “Support for the Bee Products Market.”
Author information
Authors and Affiliations
Contributions
Małgorzata Dżugan: conceptualization, methodology, writing—review and editing, funding acquisition, project administration. Michał Miłek: investigation, validation, data curation, formal analysis, writing—original draft preparation. Ewelina Sidor: investigation, visualization, data curation, writing—original draft preparation. Justyna Buczkowicz: investigation. Joanna Hęclik: investigation, resources. Aleksandra Bocian: methodology, validation, visualization, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
Małgorzata Dżugan declares that she has no conflict of interest. Michał Miłek declares that he has no conflict of interest. Ewelina Sidor declares that she has no conflict of interest. Justyna Buczkowicz declares that she has no conflict of interest. Joanna Hęclik declares that she has no conflict of interest. Aleksandra Bocian declares that she has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dżugan, M., Miłek, M., Sidor, E. et al. The Application of SDS-PAGE Protein and HPTLC Amino Acid Profiling for Verification of Declared Variety and Geographical Origin of Honey. Food Anal. Methods 16, 1157–1171 (2023). https://doi.org/10.1007/s12161-023-02489-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-023-02489-2