Abstract
The subject of this study is to determine the best solvent and optimum extraction conditions for the extraction of maximum antioxidant phenolic compounds and antioxidant activity from strawberry fruits (Fragaria x ananassa Duch.). Extractions were carried out using solvents with different polarities (water, methanol, ethanol, acetonitrile, and acetone). Box-Behnken Design was used to optimize extraction conditions, including extraction time (t), temperature (°C), and liquid/solid (L/S) ratio. In the study, extracts obtained with acetone indicated the highest total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity. The optimal extraction conditions for both responses were determined to be time of 17.5 min, temperature 52.5 °C, and liquid/solid ratio of 30:1. The maximum TPC and TFC values were found as 18.78 ± 0.22 mg of gallic acid equivalent (GAE/g) and 10.52 ± 0.35 mg of catechin equivalents (CE/g) under optimum extraction conditions. The results indicated that optimizing extraction conditions is critical for quantifying antioxidant phenolic compounds. The present model can contribute to finding a cheap way of delivering natural antioxidants in the food, cosmeceutical, and pharmaceutical industries. Furthermore, these results indicate that strawberry fruits (Fragaria x ananassa Duch.) can be a natural food colorant in dietary applications with potential health benefits.
Similar content being viewed by others
Introduction
The majority of the most significant antioxidants in the diet, such as polyphenolic chemicals and ascorbic acid, may be found naturally in fruits and vegetables (Zhang et al. 2020; Liu 2013). These substances possess many advantages including anti-aging and anti-inflammatory. Additionally, it has been suggested that they could be useful for the prevention of several chronic and neurodegenerative disorders (Zehiroglu and Ozturk Sarikaya 2019; Tulli et al. 2020; Minutti-López Sierra et al. 2019). Berry fruits play a significant role in human nutrition and provide numerous health benefits because of their high nutrient content, which includes vitamins, minerals, and bioactive components such polyphenolic phytochemicals (Olas 2018; Golovinskaia and Wang 2021; Cosme et al. 2022). Numerous epidemiological studies conducted over the past few decades have demonstrated that consuming a fruit-rich diet can lower the chance of developing a number of serious chronic diseases that affect humans (Li et al. 2013; Nani et al. 2021). Additionally, the potential health benefits of these species, primarily attributable to their phytochemical composition and antioxidant activity, have stimulated interest in research on fruit species high in polyphenols (Rudrapal et al. 2022; Karasawa and Mohan 2018; Arfaoui 2021).
One of the most widely consumed berry fruits, strawberries (Fragaria x ananassa Duch.) are mostly consumed as fresh fruit but are also used as a component in processed foods globally (Markovinović et al. 2022; Villamil-Galindo et al. 2021; Warner et al. 2021). Strawberry consumption ranks among the highest dietary sources of phytochemical antioxidants such vitamin C, carotenoids, and phenolic compounds (Šic Žlabur et al. 2020). The primary phytochemicals in strawberries are phenolic compounds, a sizable and diverse group of biologically active non-nutrients that exhibit a wide range of non-essential biological roles in plants and biologically important potential for people (Zitouni et al. 2020). Strawberry phenolics are best known, of course, for their antioxidant and anti-inflammatory properties; recently researchers found that some phenolics compounds can act as anti-COVID-19 drug agents due to their potential inhibitory activity on SARS-CoV-2 (Bhushan et al. 2021). They also have some indirect antimicrobial, anti-allergy, and anti-hypertensive properties, as well as the ability to inhibit the activity of some physiological enzymes and receptors, which helps to prevent diseases caused by oxidative stress (Afrin et al. 2016; Ganhão et al. 2019; Guiné et al. 2020). The major class of strawberry polyphenols is flavonoids, mainly anthocyanins, the most quantitatively important phenolic compounds present in strawberries in the form of pelargonidin and cyanidin derivates (Giampieri et al. 2012, Espin et al. 2000).
The phenolic compounds present in strawberries influence their quality, contributing to sensorial and organoleptic attributes, as well as to health properties (Guine et al. 2020). Strawberries are characterized by a high amount of antioxidant molecules, for example, they have a high antioxidant capacity that is closely linked to the currently available efficient oxygen radical scavengers (Biswas et al. 2019; Pukalskiene et al. 2021). To those compounds that belong to phenolics, most of which express relevant in vitro and in vivo antioxidant activities and ascorbic acid (Skrovankova et al. 2015). For use in natural antioxidants, functional foods, and nutraceuticals, discovering new and secure antioxidants and colorants from natural origin are of great interest (Babaloo et al. 2017; Espin et al. 2000; Gomes et al. 2021; Yuliati et al. 2019). One technique for evaluating the antioxidant molecules in plants is phytochemical screening (Lourenço et al. 2019).
The phenolic compounds present in strawberries (Fragaria x ananassa Duch.) as well as the used extraction techniques can both have a significant impact on the bioactivity of strawberry fruit extracts (Fierascu et al. 2020; Guiné et al. 2020; Karaaslan and Yaman 2017). Extraction is a crucial step to obtain biologically active compounds; there is no accepted technique for phenolic compound extraction. This issue arises from the wide variety of these compounds, each of which has unique physicochemical characteristics (Wang et al. 2019). These discoveries allow the promotion of strawberry fruit (Fragaria x ananassa Duch.) derivatives as potential components for cosmetic, pharmaceutical, and functional food formulations as natural colorants and antioxidants (Benchikh et al. 2020; Hoang et al. 2021; Lorenzo et al. 2017; Luzardo-Ocampo et al. 2021; Sun et al. 2013).
In this context, the phenolic compounds can be extracted using a variety of techniques, including extractions with supercritical fluids, microwaves, and ultrasounds, among others (Benchikh et al. 2020; Chaves et al. 2020; Coelho et al. 2020). Furthermore, the ultrasound-assisted extraction (UAE) is promoted as an effective, environmentally friendly, commercially viable, simple to use, and broadly applicable approach, despite the difficulties associated with its industrial scale-up (Dzah et al. 2020; Rocha et al. 2020; Koraqi et al. 2022). In general, the UAE technology efficiency can be affected by ultrasonic wave frequency, temperature, and sonication time but also by solvent characteristics; and the sample particle size also plays a role in the extraction process. The most significant important element in the UAE is the production of cavitation bubbles, which break plant tissues and release the cell’s contents. UAE typically increases the efficiency of extraction, while yielding plant derivatives that are more abundant in secondary metabolites than those obtained using alternative methods (Chemat et al. 2017; Carreira-Casais et al. 2021). Several variables, such as extraction time, temperature, extraction solvent, and solvent concentration, have an impact on the extraction of phenolic compounds from plants (Panzella et al. 2020; Patra et al. 2022). In order to achieve the highest yield, it appears important to optimize extraction conditions using the suitable solvent type for each plant sample (Zhang et al. 2018).
Designing, analyzing, and predicting extraction conditions have all been done using the response surface methodology (RSM). The conventional one-factor-at-a-time approach of optimization is neglecting the combined interaction of variables and does not guarantee obtaining optimal conditions. In order to solve this issue, statistical methodology like Box-Behnken Design (BBD), a component of RSM, has made it possible to forecast the best extraction circumstances and comprehend the relationships between extraction factors.
Therefore, the aim of the present study is to determine the best solvent and to optimize extraction conditions for extraction of maximum antioxidants phenolic compounds from strawberry fruits (Fragaria x ananassa Duch.) by using ultrasound-assisted extraction (UAE) method. The Box-Behnken design (BBD) was employed to predict the model and to optimize the extraction conditions (temperature, time, liquid/solvent ratio) based on total phenolic content, flavonoid content, and antioxidant activity.
Material and Methods
Preparation of Extracts
Strawberry fruits (Fragaria x ananassa Duch.) were obtained in June 2021 from the local market, in a maturation state ready for consumption. In the laboratory (Laboratory of Food Analysis at UBT-Higher Education Institution), they were grounded, after washing and removal of the leaves, to obtain a uniform mass of pulp. Maceration method was used for extracting phenolic compounds of strawberry fruits (Fragaria x ananassa Duch.). To define the best extraction solvent, extracts were prepared using water, methanol, ethanol, acetonitrile, and acetone. The ground fruit samples (1 g) were extracted with the appropriate solvent (30 mL) in an ultrasonic bath at 30 °C, and the mixture was vortexed for 120 min. After the extraction procedure, the mixture was centrifuged at 2000 × g for 5 min, and the supernatant was collected for further analysis. It was stored at − 4 °C until analyzed. The best solvent was determined based on maximum TPC, TFC, and antioxidant activities (DPPH radical scavenging activity, ferric reducing power).
All chemicals used in this study were analytical grade. Ethanol and methanol were provided by Alkaloid (Skopje, North Macedonia). The Folin-Ciocalteu reagent, aluminum chloride, natrium nitrite, sodium carbonate, hydrochloric acid, acetonitrile, acetone, Trolox, and gallic acid were purchased from Sigma-Aldrich (Germany).
Total Polyphenol Content Determination
Total phenolic contents of the extract of strawberry fruits were assessed by Folin-Ciocalteu reagent using the method previously reported by Singleton et al. (1999) with slight modification, with the assistance of Folin-Ciocalteu reagent by Koraqi and Lluga-Rizani (2022). Briefly, 1 mL of each fruit extract diluted 1:20 with five different concentrations (12.5, 25, 50, 100, and 200 mg/mL) of each extract (in water, methanol, ethanol, acetonitrile, and acetone) was mixed with 1 mL distilled water and 500 μL Folin-Ciocalteu reagent and stirred for 1 min. After 2 min, 4 mL of 7.5% sodium carbonate aqueous solution was added, and the mixture was incubated for 2 h at 25 °C. Standard solutions of gallic acid were used for the calibration curve. Finally, the absorbance of the mixture was measured at 765 nm by using a UV–VIS spectrophotometer GENESYS 10S™. Gallic acid was used for preparing the standard curve (0–100 mg/L), and the results were expressed as milligrams of gallic acid equivalents (GAE) per gram of fresh weight.
Flavonoid Content Determination
The total flavonoid content was measured by the aluminum chloride colorimetric assay described by de Souza et al. (2014) with some modifications. An aliquot (1 mL) of strawberry fruit extract (water, methanol, ethanol, acetonitrile, and acetone) or catechin standard solution (5, 10, 25, 50, 100, 150, and 200 mg/L) was added to a 10-mL volumetric flask containing 4 mL of water. To the flask, 0.3 mL of 5% NaNO2 and 0.3 mL of 10% AlCl3 were added. After 6 min, 2 mL of 1 M NaOH was added, and the total volume was brought to 10 mL by the addition of H2O. The solution was mixed, and the absorbance was measured by spectrophotometer at 510 nm. The total flavonoid contents of the fruits were expressed as mg catechin equivalents CE/100 g of DW. The samples were analyzed in triplicate.
DPPH Free Radical Scavenging Assay
The activity of each extract of the strawberry fruits was evaluated by modifying a DPPH method. The method was described by Brand-Williams et al. (1995) with some modifications by Rojas-Ocampo et al. (2021). Five different concentrations (12.5, 25, 50, 100, and 200 mg/mL) of each extract (in water, methanol, ethanol, acetonitrile, and acetone) were used for the determination of antioxidant activities. Each concentration of each crude extract stock solution (4 mL) was placed in a test tube, and DPPH solution (1 mL) was added to the same test tube and shaken vigorously by hand. All the test tubes were kept at room temperature in a dark place for 45 min. The control was prepared following the same procedure without adding any crude extract. The remaining purple color was measured by using a UV–VIS spectrophotometer GENESYS 10S at 520 nm after 15 min of incubation in darkness, and methanol was used as the blank. The Trolox standard curve was performed and the results expressed in mg Trolox equivalent (TEAC) per mL (mg TEAC·mL−1). The analyses were performed in triplicates.
Radical scavenging power was calculated by Eq. (1):
where Acontrol is the absorbance of DPPH solution without extract and Asample is the absorbance of the sample with DPPH solution. The half-maximal inhibitory concentration (IC50) was reported as the number of antioxidants required to decrease the initial DPPH concentration by 50%. All tests were performed in triplicate, and graphs were plotted using the average of three determinations.
Reducing Power Assay FRAP
The method described by Chu et al. (2000) with some modifications by Diem Do et al. (2014) was applied in this work to determine the reducing power of fresh fruit extract. This reducing power was investigated by observing the transformation of Fe3+ to Fe2+. The extract was diluted with distilled water (60–220 mg/mL). The diluted extract (1.0 mL) was mixed with phosphate buffer (3.5 mL, pH 6.6) and potassium ferricyanide (3.5 mL, 1%, w/w) in a test tube, followed by incubating in a water bath at 50 °C for 30 min. After the tube was removed from the water bath, trichloroacetic acid (2.5 mL, 10%, w/v) was added into the tube and centrifuged (10 min). The supernatant (3.5 mL) was diluted with distilled water (3.5 mL), and freshly prepared ferric chloride (1.0 mL, 0.1%, w/w) was added. The mixture was mixed thoroughly, and its absorbance was measured at 700 nm using a UV–VIS spectrophotometer (GENESYS 10S).
Statistical Analyses
Statistical analyses were carried out with Origin Pro. Data are expressed as the mean ± SD. The differences shown by data were significant (P < 0.05). Correlations among variables were performed with Pearson’s correlation test. Design Expert v13.0 trial software (Stat-Ease, USA) was used for the construction of experimental designs (BBD and RSA), regression analysis of experimental data, and plotting of 3D response surface graphs. One-way ANOVA was used to assess the differences among extracts obtained with different solvents, the statistical significance of the regression coefficient by F-test at 95% confidence level. The adequacy of the fitted polynomial model was expressed by the coefficient of determination (R2) and lack of fit test.
Results and Discussion
Optimization of Extraction Process
For optimization of extraction conditions using a statistical approach, three independent variables were evaluated based on total phenolic compound (TPC), total flavonoid compound (TFC), free radical scavenging assay (DPPH), and reducing power assay (FRAP) of extracts from strawberry fruits (Fragaria x ananassa Duch.) by using Box-Behnken Design. The independent variables were extraction time (A: 5–30 min), temperature (B: 30–75 °C), and liquid/solid ratio (C: 10–50). For BBD analyses, three levels as high (+ 1), medium (0), and low (− 1) were described. The code and the real value of each variable are presented in Table 1.
BBD experiments were conducted for optimization. Experimental design method was carried out by 17 experiments with five center points to test the reproducibility of the test results. Table 2 represents the designs and results of the experiment runs.
Solvent Selection for Extraction
The phenolic compounds have various chemical properties due to variations in type, number, and position of the functional groups. As a result, different solutions may have an impact on the solubility of certain molecules (Sepahpour et al. 2018). It is also reported that the polarity of solvents has a crucial role in the solubility of phenolic compounds (Babbar et al. 2014; Sepahpour et al. 2018). Therefore, as it influences the kind and quantity of phenolic chemical recovered, the choice of extraction solvent is crucial. Ethanol, methanol, and acetone which are polar organic solvents are used mostly for extraction (Borges et al. 2020). However, for pharmaceutical and food applications, using water for the extraction of bioactive chemicals might be a safer choice. This study investigates the impact of solvent type on the extraction of the phenolic compounds, using five solvents in the process: water, methanol, ethanol, acetonitrile, and acetone. Figure 1 presents the TPC, TFC, and antioxidant activities (DPPH and FRAP) of the extracts obtained with different solvents. All extracts were found to have significant total phenolic contents ranging from 10.01 ± 0.24 mg to 18.78 ± 0.22 mg GAE/g. There were significant differences in TPC and TFC of extracts obtained with different solvents (P < 0.05). Flavonoid and phenolic contents had similar extraction patterns, and acetone extract exhibited the highest amount of TPC and TFC (18.78 ± 0.22 mg GAE/g, 10.52 ± 0.35 mg QE/g, respectively). However, water extract demonstrated the lowest amount of TPC and TFC (10.01 ± 0.24 mg GAE/g, 3.25 ± 0.24 mg CE/g, respectively) (P < 0.05). Polyphenols effectively dissolved in organic solvents which are less polar than water. In the study, the differences in the phenolic content of the extracts obtained with different solvents could be attributed to solvents’ polarities. In this work, the solvents used in terms of polarity can be put in order as water > methanol > ethanol > acetonitrile > acetone. This study shows that the most efficient solvent for extraction of TPC and TFC from strawberry fruits (Fragaria x ananassa Duch.) is acetone, followed by acetonitrile, ethanol, methanol, and water.
Strawberry fruits (Fragaria x ananassa Duch.) are rich in phenolic compounds, which are responsible for some of the pharmacological activities. Our results correspond with the results of the work of Mitic et al. (2014) who reported the total phenolic content of strawberry fruit extracts in different solvents in the range of 9.59 ± 0.30 to 13.64 ± 0.10 mg GAE/g in methanolic extract, 11.53 ± 0.150 to 16.01 ± 0.36 mg GAE/g in ethanolic extract, and 14.93 ± 0.28 to 18.83 ± 0.15 mg GAE/g in acetone extract. Kajdžanoska et al. (2011) found that the total phenolic content of strawberry fruit extract in acetone extract range about 528 ± 1.5 mg GAE/100 g and in methanolic extract about 573.1 ± 1.0 mg GAE/100 g. Our results were the lowest of these studies. Gasparrini et al. (2015) noticed that the total phenolic content of strawberry fruit extract in water extract range about 2.32 ± 0.02 mg GAE/100 g, while our results also were the lowest of these studies. Our results were in accordance to those found by Mitic et al. (2014), who reported the total flavonoid content of strawberry fruit extract in different solvents in the range of 3.93 ± 0.18 to 7.05 ± 0.17 mg CE/g in methanolic extract, 4.95 ± 0.10 to 6.98 ± 0.06 mg CE/g in ethanolic extract, and 7.97 ± 0.18 to 11.85 ± 0.26 mg CE/g in acetone extract. Zitouni et al. (2020) reported that the total flavonoid content of strawberry fruit extract in water extract range from about 3.30 to 7.07 mg RE/g, and our results were similar to these studies. de Souza et al. (2014) found that the total flavonoid content of strawberry fruit extract in water extract range about 38.17 ± 2.76 mg CE/100 g, and again our results were similar to these studies.
The phenolic compounds can play an important role in neutralizing and absorbing free radicals. The effects of solvents on antioxidant activity of extracts were mostly attributed to the content change of total phenolic compounds (Che Sulaiman et al. 2017). In this study, the antioxidant activities of extracts were evaluated with antioxidant activities (DPPH and FRAP). Similar results of total antioxidant activity was reported by Mitic et al. (2014) who found that the total antioxidant activity of strawberry fruit extract in methanol extract is about 52.69 ± 1.69 μmol TE/g to 77.92 ± 2.89 μmol TE/g, 63.34 ± 1.34 μg TE/g to 86.45 ± 4.32 μg CE/g in ethanolic extract, and 89.35 ± 2.09 μg TE/g to 109.9 ± 1.21 μg TE/g in acetone extract. On the other hand, Buricova et al. (2011) reported that the total antioxidant activity by DPPH in the water extract of strawberry fruits ranged about 110.1 ± 16.6 μmol TE/g. Our results were the lowest compared to these studies. Also, the values of total antioxidant activity of strawberry fruits in acetonitrile extract are not found in the literature. Different results may be attributed to the extraction method and conditions that affect the antioxidant activities of the extract (Diem Do et al. 2014). All extracts obtained by using an organic solvent gave stronger radical scavenging capacity than that of the water extract. A similar trend was observed in the study of DPPH radical scavenging activity of strawberry fruit extract (Boeing et al. 2014; Zitouni et al. 2020; de Souza et al. 2014). The reducing power of the strawberry fruit extract, which may serve as a reflection of its antioxidant activity, was determined using a modified Fe3+ to Fe2+ reduction assay, whereby the yellow color of the test solution changes to various shades of green and blue, depending on the reducing power of the sample. Similar results are reported by Tulipani et al. (2008) who noticed FRAP for strawberry fruits in the water extract from 7.31 ± 0.09 to 14.22 ± 0.7 EqmM FeSO4/100 g. Moreover, Ganhao et al. (2019) reported FRAP values in the water extract of strawberry fruits varied from 38.2 ± 1.4 to 72.4 ± 8.1 EqmM FeSO4/100 g, and in this context, our results were the highest compared to these studies.
All these results indicate that the inconsistency in the contents of total phenols and flavonoids is affected by some factors, such as cultivars, geographical conditions, horticultural factors, the polarity of the solvents used for extraction, type of plant materials used for extraction, as well as the extraction methods.
In addition, Pearson’s correlation coefficient indicated that TPC and TFC had a positive correlation with DPPH scavenging activities (r = 0.895, 0.914; respectively, P > 0.05) and reducing power capacities (r = 0.916, 0.919, respectively; P > 0.05). Also, previous studies found a correlation between TPC with DPPH scavenging activity and reducing power capacity (Fig. 2). Phenolic compounds are majorly responsible for the antioxidant activity of plant (Zhou et al. 2021). The results of the study indicate that phenolic compounds may be the responsible for the antioxidant activity of extracts. As a result, phenolic content and antioxidant activity were observed to be affected by the solvent type. Acetone extract with the highest TPC and TFC showed the best antioxidant activity in the study. Therefore, optimization of phenolic compound extraction from strawberry fruits (Fragaria x ananassa Duch.) was carried out using acetone as solvent.
Optimization of Extraction Conditions Using Box-Behnken Design of Response Surface Methodology (RSM)
Many factors have been established to influence the extraction efficiency, such as solvent type, extraction temperature, time, and solid to solvent ratio (Che Sulaiman et al. 2017). Extraction parameters including extraction time, extraction temperature, and liquid/solid ratio were improved extraction in the next step for optimal TPC and TFC yield by BBD (Table 2). BBD was employed to define the optimum level of parameters that provided maximum total phenolic and total flavonoid extractions and to understand the relationships between the extraction parameters with TPC and TFC and antioxidant activity.
The variance analysis of the quadratic models designed for optimum TPC and TFC suggested that the models were statistically significant, as shown in Table 3 (P < 0.05). The fitted second-order polynomial equation illustrating the TPC and TFC yields by using response surface analysis is given with Eqs. 2 and 3.
The statistical significance of model terms is assumed by their respective P-value. Also, “the lack of fit F-value” is a special diagnostic test for adequacy of a model. Non-significant lack of fit is desirable. In the study, the predicted R2 (0.88 and 0.90, respectively) and adjusted R2 values for TPC and TFC (0.90 and 0.92, respectively) were in reasonable agreement with the value of R2 (0.91 and 0.92, respectively). The values of R2 imply a correlation between the experimental results and predicted values.
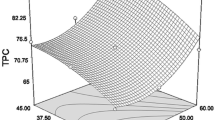
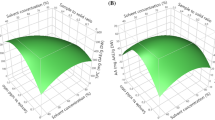
The statistical analysis indicates that the evaluated variables have a significant effect on TPC and TFC. Values of “P-value” less than 0.05 indicate that model terms are remarkable. In this case, A, B, C, A2, and B2 are significant model terms for TPC. In addition, A and C are significant model terms for TFC (Table 3). The effects of extraction time, extraction temperature, and liquid/solid ratio on TPC and TFC were illustrated in 2D contour plots and 3D response surface graphs. The 2D contour plots and 3D response surface graphical representations are shown in Fig. 3.
Figure 3 presents three-dimensional (3D) surfaces for UAE of strawberry fruits (Fragaria x ananassa Duch.). As seen in Fig. 3, the time and temperature of UAE have a predominant effect on the phenolics extraction of the current plant material, the effect of extraction temperature is positive on TPC. Considering the interactive effect of variables, the interaction between time and temperature showed a remarkable effect on TPC (P < 0.05). The TPC significantly increased with increasing extraction temperature and time. In addition, the interaction between extraction temperature and liquid/solid ratio showed a remarkably positive effect on TPC (P < 0.05), but there was no significant interaction between liquid/solid ratio and extraction time (P > 0.05) (Table 3).
Figure 4 presents the effect of extraction parameters (time, temperature, liquid/solid ratio) on TPC and TFC. Similarly, extraction time and extraction temperature profoundly influenced TFC (P < 0.05), but the liquid/solid ratio had no significant effect on TFC (P > 0.05) (Table 3). Figure 3 shows that the TFC increased in parallel to the extraction time. An increase in the yield of flavonoid was achieved with the increase of extraction time at lower temperatures. The increase in extraction temperature might be increased mass transfer improved with the penetration of solvent into the plant matrix (Chaves et al. 2020).
After optimization, an improvement in antioxidant activity was expected. The DPPH scavenging activity and ferric-reducing power capacity were found as 96.34 μmol TE/g and 107.23 EqmM FeSO4/100 g, respectively. The finding implies that optimization of extraction conditions increases phenolic compounds, which have high antioxidant capacity.
Conclusion
The results of this study indicate that solvents play an important role in the extraction, and acetone was the most efficient solvent for phenolic extraction from strawberry fruits (Fragaria x ananassa Duch.). The response surface methodology was successfully employed to optimize the extraction conditions (extraction temperature, time, and liquid/solid-ratio). It was observed that TPC yields were significantly affected by temperature, extraction time, and liquid/solid ratio, while the most important factors affecting TFC yield were extraction time and temperature. The optimal extraction conditions were found to be an extraction temperature of 17.5 °C, time of 52.5 min, and liquid/solid ratio of 30:1. We could optimize the bioactive compounds in the strawberry fruits (Fragaria x ananassa Duch.) extract obtained by ultrasonic-assisted extraction as an efficient, economically, and applicable approach. Concentrations of all phenolic compounds evaluated in this study are found to increase after optimization of extraction conditions. The study confirmed the high phenolic content of extracts from strawberry fruits (Fragaria x ananassa Duch.) parallel with high antioxidant activity that could be used as natural antioxidants and colorants in different sectors like food, cosmetic, or pharmaceutical. The results also suggest that optimizing the extraction conditions is critical for the accurate quantification of phenolic compounds in strawberry fruits (Fragaria x ananassa Duch.). The study could be extended to other plant varieties (berries, plant leaves, etc.) as rich sources of natural colorants. Furthermore, this study can be useful for the development of industrial extraction processes, to enhance the efficacy of large-scale extraction systems.
Data Availability
All the data used in the manuscript are available in the tables and figures.
Code Availability
Not applicable.
References
Afrin S, Gasparrini M, Forbes-Hernandez TY, Reboredo-Rodriguez P, Mezzetti B, Varela-López A, Giampieri F, Battino M (2016) Promising health benefits of the strawberry: a focus on clinical studies. J Agric Food Chem 64(22):4435–4449. https://doi.org/10.1021/acs.jafc.6b00857
Arfaoui L (2021) Dietary plant polyphenols: effects of food processing on their content and bioavailability. Molecules 26:2959. https://doi.org/10.3390/molecules26102959
Babaloo F, Jamei R (2017) Anthocyanin pigment stability of Cornus mas–Macrocarpa under treatment with pH and some organic acids. Food Sci Nutr 1–6. https://doi.org/10.1002/fsn3.542
Babbar N, Oberoi HS, Sandhu SK, Bhargav VK (2014) Influence of different solvents in extraction of phenolic compounds from vegetable residues and their evaluation as natural sources of antioxidants. J Food Sci Technol 51(10):2568–2575. https://doi.org/10.1007/s13197-012-0754-4
Benchikh Y, Aissaoui A, Allouch R, Mohellebi N (2020) Optimising anthocyanin extraction from strawberry fruits using response surface methodology and application in yoghurt as natural colorants and antioxidants. J Food Sci Technol. https://doi.org/10.1007/s13197-020-04710-0
Bhushan I, Sharma M,1, Mehta M, Badyal S, Sharma V, Sharma I, Singh H, Sistla S (2021) Bioactive compounds and probiotics-a ray of hope in COVID-19 management. Food Sci Human Well 10:131-140. https://doi.org/10.1016/j.fshw.2021.02.001
Biswas A, Melmaiee K, Elavarthi S, Jones J, Reddy U (2019) Characterization of strawberry (Fragaria spp.) accessions by genotyping with SSR markers and phenotyping by leaf antioxidant and trichome analysis. Sci Horticult 256. https://doi.org/10.1016/j.scienta.2019.10856
Boeing SJ, Barizão OE, Silva CB, Montanher FP, Almeida CV, Visentainer VJ (2014) Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis. Chem Cent J 8:48. https://doi.org/10.1186/s13065-014-0048-1
Borges A, José H, Homem V, Simões M (2020) Comparison of techniques and solvents on the antimicrobial and antioxidant potential of extracts from Acacia dealbata and Olea europaea. Antibiotics (basel) 9(2):48. https://doi.org/10.3390/antibiotics9020048
Brand-Williams W, Cuvellier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Science and Technology 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Buricova L, Andjelkovic M, Čermakova A, Reblova Z, Jurček O, Kolehmainen E, Verhe R, Kvasnička F (2011) Antioxidant capacity and antioxidants of strawberry, blackberry, and raspberry leaves. Czech J Food Sci 29:181–89. https://doi.org/10.17221/300/2010-CJFS
Carreira-Casais A, Otero P, Garcia-Perez P, Garcia-Oliveira P, Pereira AG, Carpena M, Soria-Lopez A, Simal-Gandara J, Prieto MA (2021) Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int J Environ Res Public Health 18(17):9153. https://doi.org/10.3390/ijerph18179153
Chaves JO, de Souza MC, da Silva LC, Lachos-Perez D, Torres-Mayanga PC, Machado APDF, Forster-Carneiro T, Vázquez-Espinosa M, González-de-Peredo AV, Barbero GF, Rostagno MA (2020) Extraction of flavonoids from natural sources using modern techniques. Front Chem. 8:507887. https://doi.org/10.3389/fchem.2020.507887
Che Sulaiman IS, Basri M, Fard Masoumi HR, Chee WJ, Ashari SE, Ismail M (2017) Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem Cent J 11(1):54. https://doi.org/10.1186/s13065-017-0285-1
Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS (2017) Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics Sonochemistry 34:540–560. https://doi.org/10.1016/j.ultsonch.2016.06.035
Chu YH, Chang CL, Hsu HF (2000) Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric 80:561–566. https://doi.org/10.1002/(SICI)1097-0010(200004)80:5%3c561
Coelho JMP, Johann G, da Silva EA, Palú F, Vieira MGA (2020) Extraction of natural antioxidants from strawberry guava leaf by conventional and non-conventional techniques. Chem Eng Commun. https://doi.org/10.1080/00986445.2020.1755658
Cosme F, Pinto T, Aires A, Morais MC, Bacelar E, Anjos R, Ferreira-Cardoso J, Oliveira I, Vilela A, Gonçalves B (2022) Red fruits composition and their health benefits—a review. Foods 11(5):644. https://doi.org/10.3390/foods11050644
de Souza VR, Aparecida P, Pereira P, Teodoro da Silva LT, Oliveira Lima LC, Pio R, Queiroz F (2014) Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem 156 362–68. https://doi.org/10.1016/j.foodchem.2014.01.125
Diem Do Q, Angkawijaya EA, Tran-Nguyen LP, Huynh HL, Soetaredjo EF, Ismadji S, Ju HY (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal 22:296–302. https://doi.org/10.1016/j.jfda.2013.11.001
Dzah CS, Duan Y, Zhang H, Wen C, Zhang J, Chen G et al (2020) The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: a review. Food Biosci 35:100–547. https://doi.org/10.1016/j.fbio.2020.100547
Espín JC, Soler-Rivas C, Wichers HJ, García-Viguera C (2000) Anthocyanin-based natural colorants: a new source of antiradical activity for foodstuff. J Agric Food Chem 48(5):1588–1592. https://doi.org/10.1021/jf9911390
Fierascu RC, Temocico G, Fierascu I, Ortan A, Babeanu NE (2020) Fragaria genus: chemical composition and biological activities. Molecules 25(3):498. https://doi.org/10.3390/molecules25030498
Ganhão R, Pinheiro J, Tino C, Faria H, Gil MM (2019) Characterization of nutritional, physicochemical, and phytochemical composition and antioxidant capacity of three strawberry “Fragaria × ananassa Duch.” Cultivars (“Primoris”, “Endurance”, and “Portola”) from Western Region of Portugal. Foods 8 (12):682. https://doi.org/10.3390/foods8120682
Gasparrini M, Forbes-Hernandez YT, Afrin S, Alvarez-Suarez MJ, Gonzàlez-Paramàs MA, Santos-Buelga C, Bompadre S, Quiles LJ, Mezzetti B, Giampieri F (2015) A pilot study of the photoprotective effects of strawberry-based cosmetic formulations on human dermal fibroblasts. Int. J. Mol. Sci 16:17870–7884. https://doi.org/10.3390/ijms160817870
Giampieri F, Tulipani S, Alvarez-Suarez MS, Quiles LJ, Mezzetti B, Battino M (2012) The strawberry: composition, nutritional quality, and impact on human health. Nutrition 28:9–19. https://doi.org/10.1016/j.nut.2011.08.009
Golovinskaia O, Wang CK (2021) Review of functional and pharmacological activities of berries. Molecules 26(13):3904. https://doi.org/10.3390/molecules26133904
Gomes J, Serrano C, Oliveira C, Dias A, Moldão M (2021) Thermal and light stability of anthocyanins from strawberry by-products non-encapsulated and encapsulated with inulin. Acta Sci Pol Technol Aliment 20(1) 79–92. https://doi.org/10.17306/J.AFS.2021.0878
Guiné RPF, Correia PMR, Ferrão AC, Gonçalves F, Lerat C, El-Idrissi T (2020) Rodrigo E. Evaluation of phenolic and antioxidant properties of strawberry as a function of extraction conditions. Braz J Food Technol 23:e2019142. https://doi.org/10.1590/1981-6723.14219
Hoang HT, Moon J-Y, Lee Y-C (2021) Natural antioxidants from plant extracts in skincare cosmetics: recent applications, challenges and perspectives. Cosmetics 8(4):106. https://doi.org/10.3390/cosmetics8040106
Jideani A, Silungwe H, Takalani T, Omolola AO, Udeh HO, Anyasi TA (2021) Antioxidant-rich natural fruit and vegetable products and human health. Int J Food Prop 24(1):41–67. https://doi.org/10.1080/10942912.2020.1866597
Kajdzanoska M, Petreska J, Stefova M (2011) Comparison of different extraction solvent mixtures for characterization of phenolic compounds in strawberries. J Agric Food Chem 59:5272–5278. https://doi.org/10.1021/jf2007826
Karaaslan NM, Yaman M (2017) Anthocyanin profile of strawberry fruit as affected by extraction conditions. Int J Food Prop 20(sup3):S2313–S2322. https://doi.org/10.1080/10942912.2017.1368548
Karasawa MMG, Mohan C (2018) Fruits as prospective reserves of bioactive compounds: a review. Natural Products and Bioprospecting 8:335–346. https://doi.org/10.1007/s13659-018-0186-6
Koraqi H, Lluga-Rizani K (2022) Effect of extraction solvent on bioactive compounds and antioxidant activity of Cichorium intybus L. grown in Kosovo. Agricult Forest 68(1):159–171. https://doi.org/10.17707/AgricultForest.68.1.09
Koraqi H, Qazimi B, Çesko C, Trajkovska Petkoska A (2022) Environmentally friendly extraction of bioactive compounds from Rosa canina L. fruits using deep eutectic solvent (DES) as green extraction media. Acta Chim Slov 69:3. https://doi.org/10.17344/acsi.2022.7559
Li C, Huang YW, Wang NX, Liu XW (2013) Oxygen radical absorbance capacity of different varieties of strawberry and the antioxidant stability in storage. Molecules 18:1528–1539. https://doi.org/10.3390/molecules18021528
Liu RH (2013) Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 4(3):384S-392S. https://doi.org/10.3945/an.112.003517
Lorenzo JM, Pateiro M, Domínguez R, Barba FJ, Putnik P, Bursać Kovačević D, Shpigelman A, Granato D, Franco D (2017) Berries extracts as natural antioxidants in meat products: a review. Food Res Int. https://doi.org/10.1016/j.foodres.2017.12.005
Lourenço SC, Moldão-Martins M, Alves VD (2019) Antioxidants of natural plant origins: from sources to food industry applications. Molecules 24(22):4132. https://doi.org/10.3390/molecules24224132
Luzardo-Ocampo I, Ramírez-Jiménez AK, Yañez J, Mojica L, Luna-Vital DA (2021) Technological applications of natural colorants in food systems: a review. Foods 10:634. https://doi.org/10.3390/foods10030634
Markovinović BA, Putnik P, Duralija B, Krivohlavek A, Ivešić M, Mandić Andačić I, Palac Bešlić I, Pavlić B, Lorenzo JM, Bursać Kovačević D (2022) Chemometric valorization of strawberry (Fragaria x ananassa Duch.) cv. 'Albion' for the production of functional juice: the impact of physicochemical, toxicological, sensory, and bioactive value. Foods 11(5):640. https://doi.org/10.3390/foods11050640
Minutti-López Sierra P, Gallardo-Velázquez T, Osorio-Revilla G (2019) Meza-Márquez OG. Chemical composition and antioxidant capacity in strawberry cultivars (Fragaria x ananassa Duch.) by FT-MIR spectroscopy and chemometrics. CyTA - J Food 17(1):724–732. https://doi.org/10.1080/19476337.2019.1645211
Mitič NM, Kostič AD, Pavlovič NA, Dimitrijevič SD. (2014) Effects of solvent extraction system on concentration and antioxidant activity of strawberry phenolics. Agro FOOD Industry Hi Tech 25(5). https://www.npao.ni.ac.rs/
Nani A, Murtaza B, Sayed Khan A, Khan NA, Hichami A (2021) Antioxidant and anti-inflammatory potential of polyphenols contained in Mediterranean diet in obesity: molecular mechanisms. Molecules 26(4):985. https://doi.org/10.3390/molecules26040985
Olas B (2018) Berry phenolic antioxidants – implications for human health? Front Pharmacol 9:78. https://doi.org/10.3389/fphar.2018.00078
Olszowy M (2019) What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol Biochem 144:135–143. https://doi.org/10.1016/j.plaphy.2019.09.039
Panzella L, Moccia F, Nasti R et al (2020) Bioactive phenolic compounds from agri-food wastes: an update on green and sustainable extraction methodologies. Front Nutr 7:60. https://doi.org/10.3389/fnut.2020.00060
Patra A, Abdullah S, Pradhan RC (2022) Review on the extraction of bioactive compounds and characterization of fruit industry by-products. Bioresour Bioprocess 9:14. https://doi.org/10.1186/s40643-022-00498-3
Pukalskiene M, Pukalskas A, Dienaite L, Revinyte S, Pereira CV, Matias AA, Venskutonis PR (2021) Recovery of bioactive compounds from strawberry (Fragaria × ananassa) pomace by conventional and pressurized liquid extraction and assessment their bioactivity in human cell cultures. Foods 10:1780. https://doi.org/10.3390/foods10081780
Rocha R, Pinela J, Abreu RMV, Añibarro-Ortega M, Pires TCSP, Saldanha AL, Alves MJ, Nogueira A, Ferreira ICFR, Barros L (2020) Extraction of anthocyanins from red raspberry for natural food colorants development: processes optimization and in vitro bioactivity. Processes 8:1447. https://doi.org/10.3390/pr8111447
Rojas-Ocampo E, Torrejón-Valqui L, Muñóz-Astecker LD, Medina-Mendoza M, Mori-Mestanza D, Castro-Alayo EM (2021) Antioxidant capacity, total phenolic content and phenolic compounds of pulp and bagasse of four Peruvian berries. Heliyon 7(8): e07787. https://doi.org/10.1016/j.heliyon.2021.e07787
Rudrapal M, Khairnar SJ, Khan J, Dukhyil AB, Ansari MA, Alomary MN, Alshabrmi FM, Palai S, Deb PK, Devi R (2022) Dietary polyphenols and their role in oxidative stress-induced human diseases: insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol 13:806470. https://doi.org/10.3389/fphar.2022.806470
Sepahpour S, Selamat J, Abdul Manap MY, Khatib A, Abdull Razis AF (2018) Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 23(2):402. https://doi.org/10.3390/molecules23020402
Šic Žlabur J, Bogdanović S, Voća S, Skendrović Babojelić M (2020) Biological potential of fruit and leaves of strawberry tree (Arbutus unedo L.) from Croatia. Molecules 25(21): 5102. https://doi.org/10.3390/molecules25215102
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Skrovankova S, Sumczynski D, Mlcek J, Jurikova T, Sochor J (2015) Bioactive compounds and antioxidant activity in different types of berries. Int J Mol Sci 16:24673–24706. https://doi.org/10.3390/ijms161024673
Sun FL, Li ZP, Li T, Fan Y, Chu Y, Tao Q (2013) Study on the extraction and stability of strawberry red pigment. Advanced Materials Research 781–784:1886–1890. https://doi.org/10.4028/www.scientific.net/amr.781-784.1886
Tulipani S, Mezzetti B, Capocasa F, Bompadre S, Beekwilder J, Ric De Vos HC, Capanoglu E, Bovy A, Battino M (2008) Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chemz 56:696–04. https://doi.org/10.1021/jf0719959
Tulli F, Lemos LM, Gutiérrez RD, Rodríguez CS, López de Mishima AB, Zanini PIV (2020) Electrochemical and spectrophotometric methods for polyphenol and ascorbic acid determination in fruit and vegetable extracts. Food technology & Biotechnology 58(2):183–91. https://doi.org/10.17113/ftb.58.02.20.6593
Villamil-Galindo E, Van de Velde F, Piagentini AM (2021) Strawberry agro-industrial by-products as a source of bioactive compounds: effect of cultivar on the phenolic profile and the antioxidant capacity. Bioresour Bioprocess 8:61. https://doi.org/10.1186/s40643-021-00416-z
Wang J, Wang J, Ye J, Vang SK, Raghavan V (2019) Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control 96:128–136
Warner R, Wu BS, MacPherson S, Lefsrud M (2021) A review of strawberry photobiology and fruit flavonoids in controlled environments. Front. Plant Sci 12:611893. https://doi.org/10.3389/fpls.2021.611893
Yuliati L, Juliana, Indrawati R (2019) Tuning the stability of red color natural pigments in fruit extracts by pH control. J Phys Conf Ser:1282-012070. https://doi.org/10.1088/1742-6596/1282/1/012070
Zehiroglu C, Ozturk Sarikaya SB (2019) The importance of antioxidants and place in today’s scientific and technological studies. J Food Sci Technol 56(11):4757–4774. https://doi.org/10.1007/s13197-019-03952-x
Zhang QW, Lin LG, Ye WC (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med 17(13):20. https://doi.org/10.1186/s13020-018-0177-x
Zhang X, Guo S, Ho TC, Bai N (2020) Phytochemical constituents and biological activities of longan (Dimocarpus longan Lour.) fruit: a review. Food Science and Human Wellness 9:95–102. https://doi.org/10.1016/j.fshw.2020.03.001
Zhou B, Jin Z, Schwarz PB, Li Y (2021) Effect of grinding and extraction conditions on the determination of antioxidant activity and phenolic acids in barley. Food Measure 15:3823–3836. https://doi.org/10.1007/s11694-021-00964-0
Zitouni H, Hssaini L, Messaoudi Z, Ourradi H, Viuda-Martos V, Hernández F, Ercisli S, Hanine H (2020) Phytochemical components and bioactivity assessment among twelve strawberry (Arbutus unedo L.) genotypes growing in Morocco using chemometrics. Foods 9:1345. https://doi.org/10.3390/foods9101345
Acknowledgements
Thanks to GAIN (Axencia Galega de Innovación) for supporting this research (grant number IN607A2019/01).
Author information
Authors and Affiliations
Contributions
H.K.: conceptualization, methodology, investigation, validation, formal analysis, writing—original draft preparation. S.A.: review and editing, final draft supervision and monitoring. A.T.P.: methodology, investigation, validation, formal analysis, contribution in writing. W.K.: review and editing, final draft supervision, and monitoring. A.S.: methodology, investigation, validation, formal analysis, contribution in writing. J.M.L.: review and editing, final draft supervision, and monitoring. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
Not applicable.
Consent to Participate
All authors have given their full consent to participate.
Consent for Publication
All authors have given their full consent for publication.
Conflict of Interest
Hyrije Koraqi declares no competing interests. Anka Trajkovska Petkoska declares no competing interests. Waseem Khalid declares no competing interests. Aqeela Sehrish declares no competing interests. Saadia Ambreen declares no competing interests. Jose Manuel Lorenzo declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koraqi, H., Petkoska, A.T., Khalid, W. et al. Optimization of the Extraction Conditions of Antioxidant Phenolic Compounds from Strawberry Fruits (Fragaria x ananassa Duch.) Using Response Surface Methodology. Food Anal. Methods 16, 1030–1042 (2023). https://doi.org/10.1007/s12161-023-02469-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-023-02469-6