Abstract
Simple, straightforward non-chromatographic method for inorganic arsenic (i-As) determination in rice using species-selective hydride generation (HG) combined with inductively coupled plasma optical emission spectrometry (ICP OES) without prior separation of methylated organoarsenicals (o-As) before measurements was developed and fully validated. i-As [As(III,V)] and o-As (DMA, MMA) species were extracted in aqua regia that oxidized As(III) to As(V), while integrity of both o-As forms was preserved. Arsenic hydrides from i-As were generated in reaction with NaBH4 (1%) in strong acidic conditions (10 mol L−1 HCl) after pre-reduction of As(V) to As(III) in a KI (0.5%)-ascorbic acid (2%)-HCl (3 mol L−1) mixture. Reactivity of As species toward HG under conditions of a rice matrix was investigated in order to improve detectability and selectivity of i-As when o-As coexists. Interferences related to contribution of DMA and MMA to the As signal recorded for i-As were pointed out. Limitations of the method for DMA and matrix effects for MMA were found advantageous to selectively measure i-As by HG-ICP OES. No matrix effects on i-As allowed to use external calibration for its quantification. Detection limit of 0.28 ng g−1 (5.6 ng g−1 in original sample), precision < 5%, and adequate accuracy (96.5–103.9%), verified by the analyte recovery study, were achieved. Applicability of the method was demonstrated by i-As determination in nine brown rice samples. Its concentrations found along with the percentage of i-As to total As (obtained after microwave digestion and HG-ICP OES detection) were similar with these reported for this type of rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although rice constitutes a staple food product for half of the world’s population due to its nutritive properties and health benefits, much interest with this food product comes from a toxicological point of view, because a rice plant can easily accumulate As into grains (Sommella et al. 2013). Toxicity of As depends on its individual species and four potentially toxic As forms, i.e., inorganic arsenicals (i-As), namely arsenite and arsenate [As(III,V)], in addition to methylated compounds, i.e., o-As, represented by dimethylarsenate and monomethylarsonate (DMA, MMA) are present in rice. Their levels are variable but i-As and DMA are predominant species (Huang et al. 2015; Narukawa et al. 2014; Nishimura et al. 2010; Raber et al. 2012). Nevertheless, despite being recognized as the minor organic form of As in rice, MMA has also been determined at very low concentrations, i.e., < 30 ng g−1 of dry weight (d.w.) (Huang et al. 2015; Petursdottir et al. 2014; Raber et al. 2012). Inorganic As, recognized as a class 1 non-threshold carcinogen, is considered much more toxic than o-As compounds; therefore, rice is the main contributor to the i-As intake among other agricultural products. Interestingly, i-As is typically found in rice produced in Asia, while DMA dominates in rice from USA. Hence, rice can be categorized into DMA (DMA > i-As) and i-As (i-As > DMA) types, and it is suggested that the DMA type is of lower risk to health (Zavala et al. 2008). Therefore, besides the total As content (t-As), knowledge regarding As species in rice, particularly hazardous i-As, is crucial to understand potential harmful effects of this foodstuff to humans. Consequently, methodologies for selective determination of i-As in rice are of a special interest. The most common speciation approach employs chromatographic separation of As species by high-performance liquid chromatography (HPLC), followed by their quantification using inductively coupled plasma mass spectrometry (ICP-MS). At present, HPLC-ICP-MS hyphenated systems, although expensive, are undoubtedly favored for As speciation analysis (Kubachka et al. 2012).
Followed the up-to-date green analytical chemistry idea to simplify the analytical chain and reduce costs and time of analysis, concurrent to HPLC-based As speciation schemes, simpler non-chromatographic methodologies, enabling determination of only the most toxic i-As in rice, were developed. Accordingly, different extraction procedures (performed with specific extracting solutions, e.g., diluted acids and bases) and isolation/separation techniques (e.g., solid-phase extraction (SPE), cloud point extraction (CPE), or dispersive liquid-liquid micro-extraction (DLLME)) combined with detection by ICP-MS, atomic absorption spectrometry (AAS), or atomic fluorescence spectrometry (AFS) have been presented to speciate As in rice (Ahmadi-Jouibari and Fattahi 2015; Chen and Chen 2014; dos Santos Costa et al. 2015; Fontcuberta et al. 2011; Huang et al. 2015; Jorhem et al. 2008; Lamont 2003; Pasias et al. 2013; Rasmussen et al. 2013). Hydride generation (HG), related to conversion of As to highly volatile hydrides in reaction with sodium tetrahydroborate (NaBH4) in acid media, in combination with atomic and mass spectrometric detectors is also a promising alternative to speciate As instead of chromatographic systems (Cerveira et al. 2015; Musil et al. 2014; Petursdottir et al. 2014; Torres-Escribano et al. 2008). This technique, originally developed to separate As from the sample matrix, was found to discriminate between various As species. HG-based differentiation of As(III), As(V), DMA, and MMA is viable because all of them react with NaBH4 with different effectiveness; hence, careful control of HG reaction conditions may result in selective generation of hydrides of individual As species (individual speciation) or species having the same nature, i.e., i-As and o-As (operational speciation) (see, e.g., Bundaleska et al. 2005; Karadjova et al. 2005; Welna and Pohl 2017).
Regarding selective i-As determination in rice, HG has been so far combined only with ICP-MS, AAS, or AFS. In some of these approaches, before HG, i-As was at first removed from o-As species by SPE with specific sorbents, i.e., silica-based, strong anion-exchange (SAX) cartridges (Chen and Chen 2014; Huang et al. 2015; Rasmussen et al. 2013). Other methods were recommended only to rice samples with no or with negligible amounts of o-As (Cerveira et al. 2015; Musil et al. 2014; Petursdottir et al. 2014; Torres-Escribano et al. 2008). In the latter case, i-As was not separated from o-As prior to its measurements under selected HG reaction conditions. Surprisingly, non-chromatographic As speciation in rice directly by HG hyphenated with ICP optical emission spectrometry (OES), to our best knowledge, has not been presented yet. In reference to this, the objective of this work was to develop and validate a dependable, quick, and robust method for operational speciation (fractionation) of As in rice using the non-chromatographic approach based on HG and ICP OES detection. We assumed that it is possible to find a unique procedure providing differentiation between i-As and o-As species in properly selected pre-reduction and HG reaction conditions and determine traces of i-As when o-As coexists by ICP OES without potential interfering effects from DMA and MMA. For that reason, behavior of all four As species, i.e., As(III,V), DMA, and MMA, during HG was examined to obtain reliable results of i-As determination. As the sample preparation step (related to reagents used) can change original speciation of As, relations between As species in properly selected optimal pre-reduction and HG reaction conditions were also examined to avoid eventual overestimation of results. Since the As response toward HG could be affected by presence of undecomposed compounds of the sample matrix extracted along with As species at the stage of sample preparation, possible interferences originating from the matrix of rice on activity of As forms under compromised reducing conditions were additionally investigated. Finally, the chosen optimal analytical proceeding that enabled to reliably differentiate between i-As and o-As and to selectively determine traces of i-As in presence of o-As was applied to analysis of nine brown rice samples.
Materials and Methods
Samples
Brown (natural) rice samples sold in Poland were analyzed. In total, nine (n = 9) samples were bought and coded as B1–B9. All of them were purchased in 400 g (4 × 100 g) packages. Prior to sampling, they were homogenized to fine powder using a planetary micromill, and then kept in closed plastic bags in dark. B1 was selected and used for optimization studies of As speciation analysis. Two reference materials (RMs), i.e., standard RM [SRM 1573a (tomato leaves)] and certificate RM [NCS ZC73036 (green tea)], were used to verify accuracy of total As determination by HG-ICP OES after microwave-assisted digestion.
Reagents and Solutions
Reagents used were of analytical grade purity. De-ionized water (18.3 MΏ cm), prepared with a Barnstead™ (USA) EASYpure RF water purification system (model D7033), was used throughout. Stock standard solutions of As(III), DMA, and MMA (1000 μg mL−1) were obtained from their respective salts (Sigma-Aldrich, St. Louis, MO, USA), i.e., sodium metaarsenite (NaAsO2), sodium cacodylate (C2H6AsNaO2 × 3H2O), and disodium methyl arsenate (CH3AsNa2O3). In case of As(V), a commercially available (1000 μg mL−1) ICP standard solution (Merck, Darmstadt, KGaA, Germany) was used. For investigations and analysis, simple aqueous and matrix-matched standard solutions were used.
A 37% (m/v) HCl solution from Sigma-Aldrich was used to acidify sample and standard solutions (to 3 mol L−1) and prepare an additional acid solution (10 mol L−1) for HG reaction. L(+)-ascorbic acid (AA, Avantor Performance Reagents, Gliwice, Poland) and KI (Avantor Performance Reagents) were used as pre-reducing agents. Single (10% (m/v) AA or 10% (m/v) KI) and mixed (KI (2.5%)-AA (10%)) solutions were prepared by dissolving respective solid reagents in de-ionized water. Sample solutions were treated with AA and KI (individually) or with their mixture to obtain required concentrations, i.e., 0.5% (m/v) (KI) and 2.0% (m/v) (AA), before final acidification to the optimum HCl concentration (3 mol L−1). A 40% (m/v) hydroxylamine hydrochloride (HH) solution, made from its solid reagent (Avantor Performance Reagents), was employed as the neutralizing agent of residual HNO3 after mineralization; it was added to sample solutions to a concentration of 2.0% (m/v) before subsequent pre-reduction.
A 65% (m/v) HNO3 (Merck) and concentrated aqua regia (AR) solutions were used for sample preparation, i.e., mineralization (for total As) and extraction (for total i-As species), respectively. AR was prepared just before use by mixing 3:1 (v/v) concentrated HCl and HNO3 solutions. Respective procedural blank sample solutions, resulted either from microwave-assisted wet digestion of 5 mL of 65% HNO3 (final mass of the digestion solution: 20.0 g) or from ultrasound-assisted extraction of 2 mL of AR (final mass of the extraction solution: 10.0 g), were prepared and used as diluents of matrix-matched standard solutions [abbreviated as HNO3-matched and AR-matched, respectively] to match the effects of these media on HG of As species. The concentration of HNO3 and AR in resulting procedural blank solutions was 3.5 and 2.5 mol L−1, respectively.
Arsenic hydrides were generated in reaction with a 1.0% (m/v) NaBH4 (Sigma-Aldrich) solution that was stabilized with 0.1% (m/v) NaOH (Sigma-Aldrich). In case of As determination from sample extracts, this NaBH4 solution contained an antifoaming agent (30% (m/v) Antifoam A aqueous emulsion from Sigma-Aldrich) to avoid extensive foam formation during HG reaction.
Experimental Procedures
Closed vessel microwave (MW)-assisted wet digestion with concentrated HNO3 and ultrasound (US)-assisted extraction with AR were applied prior to determination of t-As and selective determination of i-As (coexisted with o-As) in rice samples by HG-ICP OES, respectively. The extraction reagent and procedure employed to speciate As, i.e., differentiation between i-As (as As(V)), DMA, and MMA, was based on the sample preparation procedure developed previously for determination of t-As in crispbreads (including rice-type) (Welna 2015). In the present work, it was used to release four As species from the rice sample matrix into liquid, leading to partial conversion of As(III) into As(V) due to its oxidation, but without degradation of both o-As species into As(V).
With each set of mineralized and extracted rice samples, procedural blanks were prepared in the same way as samples and considered in final results. External calibration with HNO3-matched standards of As(V) (prepared at concentrations up to 10 ng g−1) and AR-matched standards of i-As (prepared at concentrations up to 7 ng g−1 in total of As(III,V) species) were applied to quantify contents of t-As and i-As, respectively. Additionally, by the difference between t-As and i-As contents, the content of o-As species was estimated. Samples were prepared in triplicate (n = 3).
Total Arsenic–Microwave-Assisted Closed-Vessel Wet Digestion
For MW-assisted closed-vessel wet digestion, samples of 0.5 ± 0.005 g of milled rice were mineralized with 5 mL of concentrated HNO3, employing a, recommended by the manufacturer, 9-step microwave-assisted heating program with a maximum power of 600 W for 45 min. Afterwards, resulting aliquots of samples were allowed to cool down to room temperature; after cooling and opening vessels, colorless sample digests were quantitatively transferred into 30-mL polypropylene (PP) screw-capped containers (Equimed, Poland), made up with water to 20.0 g and kept at 4 °C till analysis by HG-ICP OES.
Arsenic Speciation–Ultrasound-Assisted Extraction
For US-assisted extraction, samples of 0.5 ± 0.005 g of milled rice were weighed into 30-mL PP centrifuge tubes (Equimed, Poland), treated with 2.0 mL of AR and left to react (15 min). Resulting mixtures were sonicated in an ultrasonic bath for 15 min at ambient temperature. Next, they were diluted with water to 10.0 g and centrifuged at 12,000 rpm (10 min) to separate sample extracts from insoluble residues. Supernatants were filtered through hard filter papers (type 3H) (Munktell & Filtrak, Germany) and resulting filtrates (bright yellow) were collected into 30-mL PP screw capped containers. They were refrigerated before analysis by HG-ICP OES.
Pre-reduction of As Species
t-As and i-As were determined as As(III) in sample digests and extracts, respectively, after As(V) pre-reduction using a mixture of KI and AA in acidic medium (HCl). Final concentrations of pre-reducing agents were 0.5% (m/v) (KI) and 2.0% (m/v) (AA), while 3 mol L−1 HCl was used for acidification. Every time, pre-reduction involved 2-fold dilution. In case of i-As, adequate aliquots of each sample extract were transferred to 5-mL screw-capped PP tubes into which appropriate aliquots of concentrated solutions of AA (firstly) and KI (secondly) were added. These solutions were completed with a concentrated HCl solution to ensure required acidity, mixed, and finally left to react at room temperature for 30 min before measurements. In case of t-As, acidity of sample solutions (referred to remnants of HNO3 originating from MW digestion) must be considered at first. To prevent a negative effect of residual HNO3 (~ 1.7 mol L−1 after pre-reduction) observed in case of sample digests pre-treated with the KI-AA mixture in presence of 3 mol L−1 HCl, respective sample solutions were initially neutralized with a concentrated HH solution. Accordingly, adequate aliquots of each sample digests were transferred to 5-mL screw-capped PP tubes into which appropriate aliquots of the HH solution were added (its final concentration was 2.0% (m/v)), followed by addition of proper aliquots of the concentrated KI-AA mixture and concentrated HCl.
Importantly, for a given sample preparation procedure carried out for determination of t-As and i-As, respective blanks and matrix-matched standard solutions of As were prepared and considered in analysis.
Interference Investigations
Accuracy and selectivity of i-As determination in rice in presence of o-As extracted with the aid of AR were examined in detail. For this purpose, single (with each of As species separately) and mixed (As(III) coexisting with As(V) [abbreviated as i-As] and As(III,V) coexisting with o-As (DMA and MMA) [abbreviated as (i + o)-As]) AR-matched standard solutions (up to 20 ng g−1) were used to test selectivity of i-As determination and quantify analytical results. Concentrations of As species in respective mixed standard solutions were the same (1:1 concentration ratio). Accordingly, mixed standard solutions of i-As containing 1–10 ng g−1 of As(III,V) (that corresponded to 2–20 ng g−1 of total i-As) and mixed standard solutions of (i + o)-As containing 1–10 ng g−1 of all four As species (that corresponded to 4–40 ng g−1 of t-As) were prepared in respective AR medium. Before measurements, standards were subjected to pre-reduction with the KI-AA mixture to change pentavalent As forms into corresponding trivalent species. Calibration curves by standard additions were additionally performed for As species in order to check sample matrix interferences after extraction of selected rice (B1) with AR. Concentrations and combinations of additions of As species were similar as in those described for AR-matched standard solutions. The standard addition method was also applied (by analyte recovery test) to test selectivity of i-As determination in rice and evaluate its accuracy in presence of o-As species extracted along with i-As species using the proposed procedure. To do this, samples of B1 rice were spiked with different forms of As added separately [as i-As and o-As] or together [(i + o)-As] at 4, 8, and 12 ng g−1 of total i-As, o-As, and (i + o)-As (that corresponded do 2, 4, and 6 ng g−1 in resulting extracts after pre-reduction) and subjected to the whole US-assisted extraction procedure. Concentrations of As species in respective mixtures, i.e., As(III,V) [i-As], DMA and MMA [o-As] and As(III,V), DMA and MMA [(i + o)-As], were equal.
Arsenic Hydride Generation
Corresponding arsenic hydrides (arsines) were generated in a continuous flow system with gas-liquid phase separation. They were next directly introduced into a ICP OES spectrometer. The system consisted of a modified cyclonic spray chamber that acted as a gas-liquid phase separator, a parallel Burgener-type pneumatic nebulizer, two Y-shaped (Y) connectors, a reaction coil, and three peristaltic pumps with delivery tubes (Welna et al. 2011).
HG of As with the described manifold was proceeded identically as detailed before (Welna and Pohl 2017). Briefly, sample (S), additional acid (A), and NaBH4 (R) solutions were simultaneously pumped in separate streams using two peristaltic pumps. Solutions S and A were merged in the first Y connector before being merged with the incoming R solution in the second Y connector. The resulting reaction mixture was introduced to the spray chamber to separate volatile species from the liquid phase. Arsines were swept by a carrier Ar stream, introduced through the nebulizer gas inlet and immediately transported to the plasma torch. A post-reaction solution was drained from the spray chamber to wastes by the third peristaltic pump.
Apparatus
Measurements were performed using a Jobin Yvon (France) sequential optical emission spectrometer with radially viewed Ar-ICP, model JY 38S. Optimized operating parameters for HG reaction and ICP OES detection are listed in Table 1. Background corrected net intensities (Inet) of the As I emission line (means of three repeated measurements, n = 3) were used for all studies. Delay time (DT) necessary to achieve a steady As response was 30 s after merging acidified S and R solutions in the second Y connector. A Fritsch (Germany) planetary micromill Pulverisette 7 premium with an agate grinding bowl (20 mL) and agate balls (10 mm OD) was used to powder all rice samples. US-assisted extraction of samples was performed using a Polsonic (Warsaw, Poland) ultrasonic bath, model Sonic 14. Samples were mineralized with the aid of a Milestone (Italy) high-pressure microwave digestion system (MLS-1200 MEGA), equipped with a rotor MDR300/10 and appropriate vessels. An MPW-350 centrifuge (MPW Med. Instruments, Poland) was used to separate any solid particles from resulting AR extracts of rice samples.
Results and Discussion
Method Development
Effect of Extraction Reagent
Previously (Welna 2015), US-assisted extraction of As species from cereal-based crispbreads, carried out with the aid of a moderately concentrated AR solution and followed by generation of As hydride in reaction with NaBH4 (1.0%), after prior pre-reduction of As(V) to As(III) with KI-AA (0.5–1.5%) in HCl medium (3 mol L−1), was found reliable for determination of this element by HG-ICP OES in mentioned dairy products. Although AR led to obtain good results for As(III) and As(V), case of organic As species (DMA and MMA) was not considered in this work. Therefore, this sample preparation approach, completed with properly selected optimal pre-reduction and HG reaction conditions, was tested in the present work for non-chromatographic As speciation in rice by HG-ICP OES and extended to four As species commonly reported to be present in rice, i.e., i-As [As(III,V)] and o-As (DMA, MMA). Basically, conditions for selective determination of total i-As alone (differentiation between As(III) and As(V) excluded) in presence of o-As without prior separation of the latter species was looked for. Our recent results (Welna and Pohl 2017) showed that reactivity of all four As species differs largely versus different experimental conditions, including the NaBH4 concentration, reaction medium used for HG of As (related to type of acid and its concentration) as well as kind of the pre-reducing agent employed at the pre-reduction step. In consequence, several useful speciation procedures (SPs) using 1.0% NaBH4 (e.g., SP1 for As(III) {citrate buffer (pH 5.2) in the S solution}, SP2 for As(III) + DMA {acetate buffer (pH 4.5) in the S solution}, SP3 for As(III) + As(V) + MMA {after prior pre-reduction (0.5% KI-2.0% AA) of As species in the acidified S solution (3 mol L−1 HCl) and with the 10 mol L−1 HCl A solution for HG reaction} or SP4 for DMA + MMA {after prior pre-reduction (0.5% l-cysteine (LC)) of As species in the unacidified S solution, followed by HG in the 2 mol L−1 HCl A solution) for species-selective As determination by HG-ICP OES in one solution were proposed and verified. It has to be commented that these SPs were originally developed and recommended for simple S solutions (standards), i.e., where neither changes between As species nor any matrix effects were observed. Preliminary experiments carried out to test their applicability for simple S solutions (standards) prepared in AR proved that (i) out of four As species, i-As species (but not o-As) were conversed in this reagent (oxidation of As(III) into As(V)) and (ii) analytical results were misleading due to changes of As species reactivity in HG caused by additional acidity originating from AR. For example, it caused a significant growth of responses for As(III,V) under SP4, leading to interference effects (~ 50%) in selective determination of DMA and MMA. The effect of AR was critical for hydrides generated at SP1 and SP2 as all As species gave measurable responses; moreover, HG reaction was sudden, accompanied by extensive foam formation and required longer DT (> 2 min) to achieve steady As responses. In contrast, higher acidification of the S solution was found to be advantageous for quantification of i-As in presence of o-As species, measured under the same reaction conditions proposed for selective HG of i-As and MMA without contribution of DMA (SP3). Accordingly, this behavior of i-As in mentioned pre-reduction and HG reaction conditions was adopted here for further studies. Performance of preferred compromised pre-reduction and HG reaction conditions along with the effect of sample preparation procedure, related to extraction with AR, was verified using matrix-matched standard solutions. For this purpose, single standards (with each As species at 20 ng g−1 in 2.5 mol L−1 AR) were submitted to various experimental procedures (P), i.e., P1: [HG only (pre-reduction and acidification with HCl in the S solution omitted); HG: 1.0% NaBH4 (R)], P2: [similarly as in P1 with acidification of the S solution (3 mol L−1 HCl)], P3: [similarly as in P2 with an additional 10 mol L−1 HCl (A) solution for HG], P4: [HG with prior pre-reduction (2% AA + 0.5% KI); HG: 3 mol L−1 HCl (S), 1.0% NaBH4 (R)], and P5: [similarly as in P4 with an additional 10 mol L−1 HCl (A) solution for HG]. Appropriate S solutions for given As species (at 20 ng g−1) without AR, analogously treated and analyzed as AR-matched solutions, were taken as references. Results, expressed as Inets (mean values for n = 3 repeated measurements) along with SDs, are shown in Fig. 1.
The effect of aqua regia derived from US-assisted extraction on measurements of As species by HG-ICP OES under various pre-reduction and HG reaction conditions. Procedure 1 (P1) [HG only (pre-reduction and acidification with HCl in the S solution omitted); HG: 1.0% NaBH4 (R)]. Procedure 2 (P2) [similarly as in P1 with acidification of the S solution (3 mol L−1 HCl)]. Procedure 3 (P3) [similarly as in P2 with an additional 10 mol L−1 HCl (A) solution for HG]. Procedure 4 (P4) [HG with prior pre-reduction (2% AA + 0.5% KI); HG: 3 mol L−1 HCl (S), 1.0% NaBH4 (R)]. Procedure 5 (P5) [similarly as in P4 with the additional 10 mol L−1 HCl (A) solution for HG]. Standards: P1 (20 ng g−1), P2-P5 (2-fold dilution, 10 ng g−1). S, A, R: sample, additional acid, reductant solutions. AA: ascorbic acid
As it was expected, in case of reference standards, HG reaction carried out under conditions (P1), i.e., without any additional HCl in S solutions and with no A solution, caused that As responses from its species were not detected at all. In contrast, As hydrides could be generated directly from the S solution being only acidified with AR. Accordingly, HG reaction performed in a NaBH4-AR mixture (P1) led to similar responses of As(III), As(V), and MMA, and their signals were ~ 2.5 higher than this for DMA, suggesting differences in HG activity between i-As and o-As forms. Two-fold dilution of S solutions and their acidification to 3 mol L−1 (requirement for pre-reduction), but not subjected to pre-reduction (P2), proved integrity of these two groups of As species because As responses for i-As species were different than those obtained for o-As species. Additionally, relationships established for DMA and MMA at P2 were the same as for reference standards what indicated that original speciation of DMA and MMA was unchanged. In contrast, integrity of As(III) and As(V) was not preserved due to oxidative properties of AR, leading to oxidation of As(III) to As(V). By comparing behavior of the response measured at various HG conditions for reference standards of As(III) and As(V) before (P2, P3) and after (P4, P5) pre-reduction with this obtained for i-As forms prepared in AR, it was clear that AR medium led to complete conversion of As(III) into As(V) and enabled to determine total i-As as As(V). Addition of KI-AA to S solutions was suitable for pre-reducing As(V) to As(III) (P4, P5). Noteworthy, this mixture was found to improve the response acquired for i-As (by 23–27%) and the mentioned effect was more pronounced at a higher HCl concentration applied for HG reaction (P5). Actually, the response achieved for i-As under P5 was ~ 40% higher than that obtained without KI-AA and 10 mol L−1 HCl in the A solution (P2). Also, HG of As for i-As species was not affected in these conditions by presence of AR in the S solution; responses for As(III,V) corresponded to those recorded for reference standards of As(III) and As(V). Considering activity of DMA during HG in presence of the KI-AA mixture and high HCl concentrations (either in S or A solutions) (P4, P5), it was observed that HG for DMA could be completely suppressed in strong acidic HG reaction conditions (P5). In this sense, as compared to the response for DMA acquired for reference solutions, the more acidified S solution coming from both AR and HCl was found to be sufficient itself to deteriorate the response for DMA into nearly negligible values (P4).
Interestingly, the effect of AR was also critical for MMA pre-treated with the KI-AA mixture (P4, P5). Compared to its reference responses measured under the same reaction conditions (P4, P5), where the KI-AA mixture equalized responses for i-As and MMA, KI-AA added to the S solution had no effect on the generated MMA hydride in presence of AR. It is opposite to behavior observed for i-As species, for which complete pre-reduction of As(V) to As(III) was achieved. Hence, it was established that presence of AR in resulting sample solutions of rice is helpful for non-chromatographic speciation of this element by HG. Accordingly, initially selected pre-reduction and HG reaction conditions, as given in P5, have potential for selective determination of i-As in presence of both methylated As forms (MMA, DMA).
Chosen HG reaction conditions were similar to those previously reported (Welna 2015) (abbreviated here as P4). The difference was in overall acidity referring to the HCl concentration available for reaction with the R solution, i.e., 5.8 (P5) versus 3.0 (P4) mol L−1 (that corresponded to acidity at 6.55 (P5) and 4.25 (P4) mol L−1 when AR was included). Here, with AR present in the S solution (at concentration of 1.25 mol L−1), HG reaction carried out at higher acidity was preferred. It was responsible for complete suppression of DMA activity and leveling of i-As responses (but not MMA), conveniently resulting in lowering contribution of MMA to the overall As response coming from i-As species. The effect of AR on i-As and o-As species is very important and should be taken into account when choosing the proper calibration strategy. It appeared that calibration with simple standards could lead to erroneous results; only matrix-matched standards, i.e., with the same amount of AR as in sample extracts, are needed to consider the interfering effect coming from MMA.
Organoarsenical Interference Evaluation
As it was shown, the selected procedure for i-As determination in rice (P5), although was not applicable for DMA, the response for MMA reached in these conditions nearly 50% of this achieved for As(III,V) species. On the other hand, it should be honestly stated that As in rice is mostly present as i-As and DMA (Huang et al. 2015; Narukawa et al. 2014; Nishimura et al. 2010; Raber et al. 2012). MMA is found occasionally and its concentration is typically low (< 30 ng g−1 (d.w.)) or quite often undetectable, i.e., < 8 ng g−1 (Huang et al. 2015; Narukawa et al. 2014; Nishimura et al. 2010; Petursdottir et al. 2014; Raber et al. 2012). In this work, however, the effect of both o-As species on i-As quantification was not ignored and possible interferences coming from DMA and MMA on HG of i-As were carefully studied. To evaluate activity of individual As species in HG, sensitivity of the As line for all four As species was determined using single matrix-matched standard solutions (AR contained at 1.25 mol L−1 as in P5). Four-point calibration curves were used for this purpose, spanning the 0–10 ng g−1 concentration range of As species. Simultaneously, with mentioned calibration standard solutions, the second series of calibration standard solutions was prepared on the basis of AR extracts of rice samples (B1) and used to estimate possible interfering effects coming from sample matrix constituents extracted along with As species by AR. Mentioned extracts were spiked with known amounts of arsenicals (see the “Interference investigations” section), subjected to prior pre-reduction and then analyzed by HG-ICP OES to assess respective recoveries of As. Concentrations of additions of As species were selected considering t-As in all analyzed rice samples obtained after MW-assisted digestion, being the highest, i.e., 233 ng g−1 (d.w.) in B1. Preliminary results for US-assisted extraction with AR showed that B1 contained the total i-As content of 220 ng g−1 (d.w.). Considering the whole methodology used in this work (the sample mass and final dilution), it referred to ~ 5 ng g−1 in measured sample solutions; hence, the final concentration of added As species was in the range from 1 to 9 ng g−1 that correspond to from 40 to 360 ng g−1 of t-As in rice. Graphic visualization of given calibration curves are shown in Fig. 2. Obtained results concerning recoveries of As species are collected in Table 2.
Measured activity of As species during HG combined with ICP OES against external calibration (for AR-matched standards-the upper graph) and standard addition calibration (for the rice sample). Inet: the average net intensity of the As line for n = 3 measurements. aIII–the slope of the calibration curve for As(III). aV–the slope of the calibration curve for As(V). aM–the slope of the calibration curve for MMA
Considering external calibration [EC] (upper graph), calibration curves for As(III,V) were linear in the whole concentration range and showed high determination coefficients (R2 > 0.998). In case of MMA, the valid concentration range for which it could be accurately determined was restricted. Treatment of standards as samples after calibration allowed calculating closeness (as recovery) between measured and expected concentrations of the analyte. Accordingly, it was established that determination of MMA could not be reliable for its low concentrations, i.e., 2 ng g−1 was recovered in only 61%. For higher concentrations of MMA, i.e., > 4 ng g−1, this As species was accurately determined, i.e., recoveries varied between 95.7 and 106.0%. As expected, DMA gave no response or obtained responses were very weak and irreproducible. Sensitivities (based on slopes of calibration curves [a, in (a.u.)/(ng g−1)] for i-As species were close and nearly two times higher than those for MMA. It well agreed, taking into account different reactivities of As(III,V) and MMA under selected chemical conditions (pre-reduction and HG) (Fig. 1). Additionally, similar values of slopes for As(III) and As(V), both after KI-AA treatment, proved validity of the selected pre-reducing step. Finally, slopes obtained for external calibration (EC, see upper graph in Fig. 2) were taken for evaluation of detection (DLs) and quantification (QLs) limits of As species, which were calculated in ng g−1 using 3σ (DL) and 10σ (QL) criterion. DLs and QLs for As(III) and As(V) were practically the same, i.e., 0.29 (DL) and 0.95 ng g−1 (QL). Those for MMA were higher and equaled 0.52 (DL) and 1.7 ng g−1 (QL), what explained difficulties with adequate determinations of low amounts of this As form.
Considering calibration curves originating from the standard addition method [SC], it was found that the sample matrix arising from extraction of rice significantly affected the HG process of MMA to such an extent that concentrations up to 3 ng g−1 of added MMA to sample solutions were too low to be measured. As can be seen in Fig. 2, measurable MMA responses (i.e., 13–34% of those recorded for As(III,V)) were obtained when this form was present at a concentration higher than 3.6 ng g−1, what was probably involved with a higher QL value of MMA due to matrix effects. Truly, interfering effects on measurements of MMA were responsible for a 2.7-fold lower slope of the SC curve than this achieved for the EC curve with AR-matched standards. Consequently, DL and QL values of MMA calculated on the basis of SC were much higher, i.e., 0.79 and 2.6 ng g−1, respectively. These results clearly showed that extraction with AR as the sample preparation procedure of rice followed by analysis of resulting sample extracts on the MMA content against SC is required to obtain reliable (true and precise) results (Table 2). Recoveries of MMA were established to vary from 0% (1–3 ng g−1) through 80.5% (3.5 ng g−1) to 96.1–109.5% (4–9 ng g−1). By using EC, recoveries of MMA were poorer, i.e., 23.3% (3.5 ng g−1) and 37.2–61.4% (4–9 ng g−1). As previously, As hydrides for DMA were not generated. Apparently, there was no As response for this species when measuring respective spiked sample solutions. In case of both i-As species, slopes of SC curves were substantially the same as those achieved for EC curves, what indicated that (i) the rice matrix does not interfere with As(III,V) determinations, (ii) i-As can be determined using EC, and (iii) calibration standard solutions can be prepared using either As(III) or As(V). Respective recoveries obtained against EC and SC were very high and varied for As(III) between 100.0–107.3% (EC) and 97.6–103.3% (SC), while for As(V), it was 95.8–104.6% (EC) and 92.8–104.3% (SC). It must be commented that in order to completely exclude or have a very low interfering effect from MMA, determination of i-As in rice against EC is favorable and has to be selected to obtain reliable results of speciation analysis.
Regarding activity of both o-As species in HG under pre-reduction and HG reaction conditions corresponding to P5, it appeared that selective determination of total i-As in S solutions of rice containing MMA and DMA was possible. No interferences from MMA on i-As were noted for S solutions containing up to 3.6 ng g−1 of MMA. Considering the sample mass taken for extraction in AR and final dilution of analytes, it indicated that rice samples with MMA up to 160 ng g−1 (d.w.) can be analyzed on the content of i-As with no contribution of MMA to final results of total As(III,V). In contrast, interferences from DMA on i-As were insignificant independently of its concentration in S solutions of rice.
In the following assay, analogously to separately measured As standards, determination of total i-As in presence of both o-As species was investigated. The main goal was to keep the o-As/i-As ratio (related to contribution of o-As to response of i-As) as low as possible in order to achieve discrimination between both group of As species and possibility of selective determination of i-As in rice with the proposed procedure (P5). Mixed AR-matched standards of i-As [As(III) coexisted with As(V)] and (i + o)-As [As(III,V), DMA, and MMA], containing the same concentrations of these species in respective mixtures (at 1:1 ratio), i.e., 1–9 ng g−1 for each species that corresponded to 2–18 ng g−1 of total i-As and 4–36 ng g−1 of total (i + o)-As), were analyzed. Results that were obtained, concerning two sets of EC curves with 7 mixed AR-matched standard solutions of i-As and (i + o)-As, and another set of SC curves based on 7 standard additions to AR extracts of rice (B1), are demonstrated in Fig. 3. In this way, these graphs (i) show any potential interferences of o-As on i-As versus total i-As determined in the mixture and (ii) give information about the influence of the rice matrix on HG of As species.
The effect of methylated As species (o-As) on determination of i-As by HG-ICP OES considering external calibration with AR-matched standards (the upper graph) and standard addition calibration (for the rice sample). Inet: the average net intensity of the As line for n = 3 measurements. i-As–the sum of inorganic As species [As(III) and As(V)] at 1:1 concentration ratio. (i + o)-As–the sum of inorganic and organic As species [As(III) + As(V) + DMA + MMA] at 1:1 concentration ratio
Data achieved for i-As with EC and SC were in good agreement. In both cases, linearity of calibration curves up to 18 ng g−1 of total i-As with high determination coefficients (R2 > 0.999) was obtained. Sensitivity values, i.e., slopes of calibration curves were the same, i.e., 10.82 (a.u.)/(ng g−1) for EC and 10.81 (a.u.)/(ng g−1) for SC, and moreover, in accordance with separately measured As(III) and As(V) standards (Fig. 2). Accordingly, DLs and QLs for As(III) and As(V) calculated here for rice samples were the same too. It shows that in the proposed methodology for selective determination of total i-As in rice, there was practically no effect from the sample matrix. As a result, quantitative recoveries of added i-As were obtained, i.e., 95.6–103.3% (Table 3), justifying the use of EC for quantification of i-As.
Compared with i-As calibration curves, linearity of those related to mixed standards (i-As coexisted with o-As) was limited and depended on concentrations of both methylated As forms. Accordingly, slopes of (i + o)-As calibration curves were similar and corresponded to slopes of i-As calibration curves, i.e., 10.74 (a.u.)/(ng g−1) for EC and 10.83 (a.u.)/(ng g−1) for SC, confirming absence of interferences coming from the rice matrix, and satisfactory R2 coefficient of 0.999. However, it was valid only in the range of 0–7 ng g−1 of total i-As in the mixture. Assuming that i-As was recovered in 100%, there was no recovery of o-As in these conditions. As it was predicted (Fig. 2), at concentrations of total o-As higher than 7 ng g−1, (i + o)-As calibration curves were no longer linear (Fig. 3) due to increasing activity of methylated species (particularly MMA) in HG reaction. Moreover, considering tolerance to the real sample matrix, behavior of o-As species was close to this observed using single standards. Accordingly, the difference between responses of i-As related to presence of o-As was higher (~ 30% increase) when using EC than this observed in case of SC (~ 17% increase). These differences were about 50% poorer than those achieved when using single standards, i.e., 56% for EC and 29% for SC. Since DMA did not contribute to the response of i-As in pre-reduction and HG reaction conditions of P5, only growing activity associated with presence of MMA may lead to overestimation of the i-As response measured for sample extracts. Results given in Table 3 show that the calculated percent error for i-As determination in rice due to interferences from o-As (each As species in an equal concentration > 3.5 ng g−1) and using EC varied between 15 and 18%. Calculations made for i-As with MMA at the 1:1 concentration ratio gave the error between 29 and 36%, being in agreement with the effect of MMA on the response of As(III,V) acquired for single standards.
Concluding, the proposed methodology (P5) enables selective determination of i-As and may be applied to fractionation analysis of rice containing methylated As species or not. DMA (independently of its concentration) will not contribute to quantification of i-As. Quantified concentrations of i-As may be overestimated when MMA is present in rice at > 140 ng g−1 (d.w.) that corresponds to > 3.5 ng g−1 in respective sample extracts). However, such situation is never found in rice of different origin.
Verification of Proposed Procedure
Samples of B1 were analyzed for total i-As with the proposed procedure (P5) against both SC and EC using AR-matched calibration standards to choose the best quantification strategy. Concentration ranges of standards were preferentially limited to avoid the possible negative effect from o-As.
There was generally good agreement between the total i-As concentration determined using either single [As(III,V)] or mixed [i-As and (i + o)-As] calibration standard solutions at concentration ranges of 0–9 [As(III) or As(V)], 0–7 [total i-As], and 0–14 ng g−1 [total As, i.e., the sum of i-As and o-As] (Fig. 4a). Additionally, the rice matrix did not affect results of analysis; hence, i-As could be determined using EC. Importantly, no deterioration of results of i-As determination was observed when (i + o)-As calibration standards were used, likely due to no contribution of MMA and DMA to HG and the overall As response. As the sum of As(III) and As(V), i.e., total i-As, was determined here, mixed matrix-matched standard solutions of both species in the range of 0–7 ng g−1 (total i-As) were chosen for EC. Under optimal experimental conditions, related to pre-reduction and HG reaction, DL and QL values for i-As in the sample solution were 0.28 and 0.94 ng g−1, respectively. Taking into account the sample mass and final dilution employed in the selected procedure (P5), it corresponded to the DL of 5.6 ng g−1 in the original sample that is adequate for trace analysis. Precision of replicated (n = 5) measurements of i-As standard solutions (4 ng g−1), expressed as relative standard deviation (%RSD), was 2.7%, pointing satisfactorily repeatability. For the real rice matrix (B1) containing ~ 220 ng g−1 of i-As, precision was also good (3.2%, n = 3). Accuracy of the proposed method was verified by the recovery test. Samples of B1 were spiked before preparation with i-As (i), o-As (ii), and (i + o)-As (iii) to obtain total of 2, 4, and 6 ng g−1 in measured solutions (as detailed in the “Interference investigations” section). Quantitative recoveries of added i-As were obtained, i.e., 96.5–103.9% (i, iii), while those coming from o-As (ii) were equal to zero. In the third case, i.e., for i-As with coexisted o-As(iii), recoveries of o-As were zero too. It evidenced absence of losses of i-As and any interfering effects from the rice matrix on measurements of i-As. It also proved integrity of As species, i.e., i-As and o-As during all steps of analysis, guarantying selective determination of i-As in presence of o-As.
Comparison of the average i-As concentration (n = 3) determined in rice (B1) by HG-ICP OES against external calibration (EC) and standard addition calibration (SC) regarding standard composition (single [As(III,V)] or mixed [i-As and (i + o)-As]) and their concentration range. a Selective i-As determination. b Nonselective i-As determination (with interference from o-As). i-As–the sum of inorganic As species [As(III) and As(V)] at 1:1 concentration ratio. (i + o)-As–the sum of inorganic and organic As species [As(III) + As(V) + DMA + MMA] at 1:1 concentration ratio
Undoubtedly, as evidenced in this work, MMA concentrations < 3.5 ng g−1 in the S solution appeared to be harmless for selective determination of i-As (Fig. 4a). Nonetheless, the effect of MMA on i-As quantification was further examined, assuming (hypothetically) presence of MMA in rice, to show that it could be a factor affecting accuracy of analysis, i.e., leads to (i) unreliable results of the i-As determined against EC or (ii) underestimation of i-As content determined against SC. For this purpose, mixed standard solutions of i-As and o-As [(i + o)-As] in ranges of 0–18, 0–22, and 0–36 ng g−1 of t-As (with equal concentration of each As species in respective mixtures) were used to quantify the i-As concentration in rice using EC and SC. Simultaneously, quantifications based on i-As standards (without o-As) at given concentration ranges, i.e., 0–9, 0–11, and 0–18 ng g−1 of total i-As, with both EC and SC were taken as reference. Unfortunately (Fig. 4b), the increase in the MMA concentration > 3.5 ng g−1 in respective mixtures made difficulties in i-As quantification against EC with AR-matched standards because linearity of the (i + o)-As curve failed (R2 < 0.96). Otherwise, application of SC resulted in a gradual decrease in the i-As content (by 16–39%) as the concentration of MMA in the S solution increased. As expected, such problems were not observed when calibration with i-As standards was applied. Using EC or SC, accurate results were always achieved. Nevertheless, evaluation of interferences from MMA (related to its activity in HG) is critically required to obtain dependable results.
Application
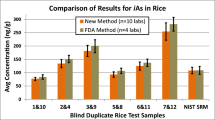
The proposed method was applied to analysis of nine brown rice samples, and results are summarized in Table 4. Data for t-As and i-As are means (n = 3) along with SDs. Sum of o-As species were estimated by the difference between t-As and i-As contents. Additionally, percent ratios of i-As to t-As were calculated and included in this table. Accuracy of determination of t-As by HG-ICP OES after MW-assisted digestion was verified by analyzing two RMs with assigned values of 0.112 ± 0.004 μg g−1 [SRM 1573a (tomato leaves)] and 0.270 ± 0.05 μg g−1 [NCS ZC73036 (green tea)]. Determined t-As concentrations, i.e., 0.111 ± 0.009 μg g−1 (SRM 1573a) and 0.264 ± 0.03 μg g−1 (NCS ZC73036), well corresponded to certified values at a 95% confidence level, as established using the t test.
t-As determined in analyzed brown rice samples varied between 172 and 233 ng g−1. The concentration of i-As ranged from 68 to 221 ng g−1, giving its contribution of 79 ± 18% to t-As. This pointed out that As contained in analyzed brown rice samples was represented mainly by its inorganic species. Therefore, they could be categorized to the i-As rice type. The only one exception was rice B6, in which i-As was lower than 40% of t-As. Obtained results were within concentration ranges reported for this type of rice (Cerveira et al. 2015; Chen and Chen 2014; dos Santos et al. 2013; Fontcuberta et al. 2011; Jorhem et al. 2008; Kuramata et al. 2011; Llorente-Mirandes et al. 2012; Narukawa et al. 2014; Nookabkaew et al. 2013; Pasias et al. 2013; Petursdottir et al. 2014; Rasmussen et al. 2013; Seyfferth et al. 2014; Shraim 2017; Sun et al. 2008; Torres-Escribano et al. 2008). The remaining As content, provided by organic As species, varied between 12 and 112 ng g−1. These concentrations were below the QL of o-As, hence, would not affect quantification of i-As with the proposed method. In view of this, the way to evaluate the concentration of o-As, i.e., by the difference between t-As and i-As concentrations, was highly justified.
Conclusions
As shown in this work, hydride generation (HG) with ICP OES detection, combined with adequate sample preparation, has a potential for selective determination of i-As in rice in presence of other organoarsenic compounds. We developed dependable non-chromatographic method based on ultrasound (US)-assisted extraction (15 min, room temperature) of four As species, i.e., i-As [As(III,V)] and o-As (DMA, MMA) with aqua regia (AR), followed by virtual separation of i-As from DMA and MMA under species-selective HG for i-As (achieved at strongly acidic conditions (10 mol L−1 HCl) and As(V) pre-reduction to As(III) with the 0.5% KI-2% ascorbic acid mixture in 3 mol L−1 HCl), and ended by detection of i-As by ICP OES. Activity of o-As in HG strongly depended on reducing conditions (DMA) and interfering effects coming from the rice matrix (MMA). Nevertheless, contribution of DMA and MMA to the quantified i-As concentration was negligible; hence, separation of o-As from i-As prior to measurements of the latter species was possible. Overestimation of the i-As concentration due to MMA could only occur when its content in rice would be > 140 ng g−1 (d.w.). Such situation, however, is not found in rice. Satisfactorily, HG of i-As was not influenced by the rice matrix and external calibration (EC) could be recommended for this analysis. Under optimal conditions, selective, precise (RSD < 5%), accurate (96.5–103.9% as recoveries), and sensitive (DL at 0.28 ng g−1 [5.6 ng g−1 (d.w.)] determination of i-As was reached. Finally, it was successfully applied to several brown rice samples and the i-As concentration within 172–233 ng g−1 was determined. By comparison with the total As (t-As) content, determined by HG-ICP OES after microwave-assisted digestion, it was concluded that proportion of i-As in examined rice samples varied between 38 and 95%. The proposed method was simple, safe, and incurred lower operation and maintenance costs than those required during As speciation by HPLC-ICP-MS. We also believe that it could be suitable for analysis of traces of i-As in other cereals or food product of cereal origin.
References
Ahmadi-Jouibari T, Fattahi N (2015) Speciation of inorganic species and total inorganic arsenic in rice using microwave-assisted dispersive liquid-liquid micro-extraction and electrothermal atomic absorption spectrometry. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32:1140–1147
Bundaleska JM, Stafilov T, Arpadjan T (2005) Direct analysis of natural waters for arsenic species by hydride generation atomic absorption spectrometry. Int J Environ Anal Chem 85:199–207
Cerveira C, Pozebon D, Pompeu de Moraes D, Silva de Fraga JC (2015) Speciation of inorganic arsenic in rice using hydride generation atomic absorption spectrometry (HG-AAS). Anal Methods 7:4528–4534
Chen G, Chen T (2014) SPE speciation of inorganic arsenic in rice followed by hydride-generation atomic fluorescence spectrometric quantification. Talanta 119:202–206
dos Santos Costa BE, Coelho NMM, Coelho LM (2015) Determination of arsenic species in rice samples using CPE and ETAAS. Food Chem 178:89–95
dos Santos WNL, Cavalcante DD, Macedo SM, Nogueira JS, da Silva EGP (2013) Slurry sampling and HG AFS for the determination of total arsenic in rice samples. Food Anal Methods 6:1128–1132
Fontcuberta M, Calderon J, Villalbi JR, Centrich F, Portana S, Espelt A, Duran J, Nebon T (2011) Total and inorganic arsenic in marketed food and associated health risks for the Catalan (Spain) population. J Agric Food Chem 59:10013–10022
Huang Y, Shan J, Fan B, He Y, Xia S, Sun Y, Lu J, Wang M, Wang F (2015) Determination of inorganic arsenic in rice by solid phase extraction and hydride generation atomic fluorescence spectrometry. Anal Methods 7:8896–8900
Jorhem L, Astrand C, Sundstrom B, Baxter M, Stokes P, Lewis J, Grave KP (2008) Elements in rice from the Swedish market: 1. Cadmium, lead and arsenic (total and inorganic). Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25:284–292
Karadjova IB, Lampugnani L, Onor M, D’Ulivo A, Tsalev DL (2005) Continuous flow hydride generation-atomic fluorescence spectrometric determination and speciation of arsenic in wine. Spectrochim Acta B 60:816–823
Kubachka KM, Shockey NV, Hanley TA, Conklin SD, Heitkemper DT (2012) FDA method EAM 4.11
Kuramata M, Abe T, Matsumoto S, Ishikawa S (2011) Arsenic accumulation and speciation in Japanese paddy rice cultivars. Soil Sci Plant Nutr 57:248–258
Lamont H (2003) Concentration of inorganic arsenic in samples of white rice from the United States. J Food Compos Anal 16:687–695
Llorente-Mirandes T, Calderon J, Lopez-Sanchez JF, Centrich F, Rubio R (2012) A fully validated method for the determination of arsenic species in rice and infant cereal products. Pure Appl Chem 84:225–238
Musil S, Petursdottir AH, Raab A, Gunnlaugsdottir H, Krupp E, Feldmann J (2014) Speciation without chromatography using selective hydride generation: inorganic arsenic in rice and samples of marine origin. Anal Chem 86:993–999
Narukawa T, Matsumotto E, Nishimura T, Hioki A (2014) Determination of sixteen elements and arsenic species in brown, polished and milled rice. Anal Sci 30:245–250
Nishimura T, Hamano-Nagaoka M, Sakakibara N, Abe T, Maekawa Y, Maitani T (2010) Determination method for total arsenic and partial-digestion method with nitric acid for inorganic arsenic speciation in several varieties of rice. Food Hyg Safe Sci (Shokuhin Eiseigaku Zasshi) 51:178–181
Nookabkaew S, Rangkadilok N, Mahidol C, Promsuk G, Satayavivad J (2013) Determination of arsenic species in rice from Thailand and other Asian countries using simple extraction and HPLC-ICP-MS analysis. J Agric Food Chem 61:6991–6998
Pasias IN, Thomaidi NS, Piperaki EA (2013) Determination of total arsenic, total inorganic arsenic and inorganic arsenic species in rice and rice flour by electrothermal atomic absorption spectrometry. Microchem J 108:1–6
Petursdottir AH, Friedrich N, Musil S, Raab A, Gunnlaugsdottir H, Krupp EM, Feldmann J (2014) Hydride generation ICP-MS as a simple method for determination of inorganic arsenic in rice for routine biomonitoring. Anal Methods 6:5392–5396
Raber G, Stock N, Hanel P, Murko M, Navratilova J, Francesconi KA (2012) An improved HPLC-ICPMS method for determining inorganic arsenic in food: application to rice, wheat and tuna fish. Food Chem 134:524–532
Rasmussen RR, Qian Y, Sloth JJ (2013) SPE HG-AAS method for the determination of inorganic arsenic in rice-results from method validation studies and a survey on rice products. Anal Bioanal Chem 405:7851–7857
Seyfferth AL, McCurdy S, Schaefer MV, Fendorf S (2014) Arsenic concentrations in paddy soil and rice and health implications for major rice-growing regions of Cambodia. Environ Sci Technol 48:4699–4706
Shraim AM (2017) Rice is a potential dietary source of not only arsenic but also other toxic elements like lead and chromium. Arab J Chem 10:S3434–S3443
Sommella A, Deacon C, Norton G, Pigna M, Violante AA, Meharg A (2013) Total arsenic, inorganic arsenic, and other elements concentrations in Italian rice grain varies with origin and type. Environ Pollut 181:38–43
Sun GX, Williams PN, Carey AM, Zhu YG, Deacon C, Raab A, Feldmann J, Islam RM, Meharg AA (2008) Inorganic arsenic in rice bran and its products are an order of magnitude higher than in bulk grain. Environ Sci Technol 42:7542–7546
Torres-Escribano S, Leal M, Velez D, Montoro R (2008) Total and inorganic arsenic concentrations in rice sold in Spain, effect of cooking, and risk assessments. Environ Sci Technol 42:3867–3872
Welna M (2015) Determination of trace arsenic content in commercial crispbread by hydride generation inductively coupled plasma optical emission spectrometry. Aust J Chem 68:441–446
Welna M, Pohl P (2017) Potential of the hydride generation technique coupled to inductively coupled plasma optical emission spectrometry for non-chromatographic as speciation. J Anal At Spectrom 32:1766–1779
Welna M, Lasowska J, Zyrnicki W (2011) Determination of some inorganic species of Fe, Mn and Cr by chemical vapor generation hyphenated with inductively coupled plasma atomic emission spectrometry. J Braz Chem Soc 22:1164–1167
Zavala YJ, Gerads R, Gorleyok H, Duxbury JM (2008) Arsenic in rice: II arsenic speciation in USA grain and implications for human health. Environ Sci Technol 42:3861–3866
Funding
This study was financed by a statutory activity subsidy from Polish Ministry of Science and Higher Education for the Faculty of Chemistry, Wroclaw University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Maja Welna declares that she has no conflict of interest. Pawel Pohl declares that he has no conflict of interest. Anna Szymczycha-Madeja declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Welna, M., Pohl, P. & Szymczycha-Madeja, A. Non-chromatographic Speciation of Inorganic Arsenic in Rice by Hydride Generation Inductively Coupled Plasma Optical Emission Spectrometry. Food Anal. Methods 12, 581–594 (2019). https://doi.org/10.1007/s12161-018-1388-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1388-6