Abstract
The use of proton transfer reaction mass spectrometry (PTR-MS) for freshness classification of chicken and turkey meat samples was investigated. A number of volatile organic compounds (VOCs) were selected based on the correlation (> 95%) of their concentration during storage at 4 °C over a period of 5 days with the results of the microbial analysis. In order to verify if the selected compounds are not sample-specific, a number of samples sourced from various retailers were classified using the concentration of these compounds in the samples’ volatile fraction as input variables. The classification was performed using the support vector machines (SVM) supervised pattern recognition algorithm. It was concluded that it is possible to evaluate the shelf life of meat samples obtained from the same source based on the results of a prior analysis. The PTR-MS fingerprint approach might supplement the currently used methods of shelf life evaluation of poultry due to the short time and non-destructive nature of measurement and ease of quantitative analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The shelf life of poultry is determined by numerous factors, in particular, the number of microorganisms which cause biodeterioration of the product. Good hygienic conditions and refrigeration are necessary to delay the onset of bacterial spoilage. Also, the use of modified atmosphere packing (MAP) allows prolonging the period of time in which the product’s freshness is preserved (Jeremiah 2001). However, a large proportion of fresh poultry is still distributed at low storage temperatures without packaging (Cooksey 2014). Currently, the most commonly used method for determination of meat status with respect to spoilage is the analysis of total viable bacteria counts (TVC) which requires an incubation period of up to 72 h for colony formation (Mayr et al. 2003a). The analysis of the volatile fraction of a meat sample is a more rapid approach. It can be based on the identification of volatile compounds as is the case with gas chromatography-mass spectrometry (GC-MS) (Wojnowski et al. 2017a). However, since the composition of fresh poultry’s volatile fraction can be influenced by several variables, a more holistic approach in which the flavour profile of a sample is analysed might prove more convenient for fast determination of the product’s freshness (Wojnowski et al. 2017b). This can be realised using proton transfer reaction mass spectrometry (PTR-MS) which can be used to measure rapidly mixtures of volatiles with high sensitivity and to determine their concentration in real time without the need for extensive sample preparation. This, combined with high time resolution and the possibility of online monitoring of the time-intensity evolution of sample’s headspace composition, makes this technique particularly useful in food analysis. Furthermore, since virtually no sample preparation is required, the measured mass spectra closely reflect genuine distributions of volatiles in the headspace (Mayr et al. 2003b). The method itself is based on soft ionisation of volatiles, usually volatile organic compounds (VOCs) through reaction with hydronium ions. A majority of common VOCs react with H3O+ ions, while other main components of ambient air do not (Mayr et al. 2003b). The technique was described in detail by Jordan et al. (Jordan et al. 2009).
When assessing meat shelf life with regard to food spoilage the most important information is binary—the sample can be classified as either ‘fresh’ or ‘suspect’. To this end, the mass spectrum of the sample’s headspace obtained using PTR-MS can be analysed using supervised pattern recognition methods (Biasioli et al. 2011). This allows to rapidly assess the products’ freshness without the need for determination of particular components of the gaseous mixture. Such approach might prove particularly useful in screening and serial analysis of large numbers of samples. However, care should be taken when treating the entire mass spectrum of the sample’s volatile fraction as an input for statistical analysis. When the ratio of the number of independent measurements to the number of variables is low there is a chance that a coincidental correlation between a random variable and, e.g. poultry shelf life shall occur (Vul et al. 2009). The ability of PTR-TOF-MS to measure a large number of volatile compounds increases the likelihood of the occurrence of these so-called ‘voodoo correlations’ (Amann et al. 2014). For that reason, it might prove necessary to curtail the number of mass peaks used as independent variables for chemometric analysis based on reference criteria.
In this paper, the validity of an approach in which VOCs detected in the poultry sample’s headspace are selected based on the correlation of the changes in their concentration during cold storage with a reference measurement of TVC prior to classification using supervised pattern recognition techniques is verified. In order to determine whether the selected compounds are not sample-specific a number of samples sourced from various retailers were classified using these compounds as input variables. The ‘fingerprinting’ approach coupled with chemometrics is used to drastically shorten the time of a single analysis which is important when considering the future implementation of the technique in the industry.
Materials and Methods
Sample Preparation
Fresh poultry meat was procured in various local distribution centres in Gdańsk, Poland. In total, five separate samples were procured: two samples of chicken meat from the same supplier 2 weeks apart (samples 1a and 1b), samples of chicken meat from two additional suppliers (samples 2 and 3) and a sample of turkey meat from a fourth supplier (sample 4). In each case, the declared shelf life was 2 days and the animals were slaughtered in the evening on the day preceding the first day of the analysis. They were then transported under refrigeration to distribution centres, where the carcasses were dismembered. Breast muscles from two different birds were then ground together in a thoroughly cleaned industrial grinder and transported in a portable cooler to the laboratory on the morning of the first day of the experiment. Samples weighing 4 g were placed in 20 cm3 headspace vials, covered with polyethylene foil and refrigerated at 4 °C. The samples of meat from each retailer were analysed over a period of 5 consecutive days, with new samples used for each analysis, with a total of 225 samples.
Microbiological Analysis
Meat samples (4 g) were placed in 50 ml conical centrifuge tubes which were then filled to the volume of 40 ml with a sterile solution consisting of 2.2% peptone (Sigma-Aldrich, Steinheim, Germany), 1.1% NaCl (POCH, Gliwice, Poland) and 2.2% glucose (Sigma-Aldrich, Steinheim, Germany) and centrifuged for 2 min. Decimal dilutions in peptone solution were prepared in triplicate and 0.1 ml samples of appropriate dilutions were poured on plate count agar (PC, BTL, pH = 7.2) to determine total viable bacteria counts. Bacteria were enumerated at 30 °C for 72 h. The analysis was carried out according to the procedure outlined in ISO 4833:2013.
PTR-MS Analysis
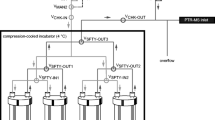
In order to analyse the VOCs in the meat samples’ headspace the proton transfer reaction mass spectrometer with time-of-flight analyser PTR-TOF 1000 Ultra (Ionicon GmbH, Innsbruck, Austria) was used. The system allows an on-line measurement of components with concentrations in the range of several pptv with high sensitivity (> 500 cps/ppbv). The use of time-of-flight analyser facilitates screening analysis without the decrease of detection parameters. Prior to each analysis, vials were sealed with caps lined with a silicon-PTFE membrane and incubated at 30 °C for 10 min. Their headspace was drawn at 50 cm3∙min−1 through a transfer line heated to 70 °C into the PTR-MS system. Ambient air passed through a carbon filter (Supelpure HC, Supelco, Sigma-Aldrich, Steinheim, Germany) was used as carrier gas. Mass spectra were collected over a range of masses with m/z values of 30 to 200 Da every second for a total of 120 spectra per analysis. Background concentrations were detected before each analysis by measuring the VOCs from an empty vial incubated in parallel with the sample and subtracted from the measured concentrations. The E/N ratio was set to 130 Td which allowed for ionisation of a wide range of chemical compounds. Higher E/N values might lead to higher fragmentation while lower values could result in increased formation of water/hydronium ion clusters which would, in turn, hinder data interpretation (Lindinger et al. 1998). IoniTOF v. 2.4.40 software was used to record the spectra and PTR-MS Viewer v. 3.2.3.0 was used to process the data. A schematic representation of the experimental setup is shown in Fig. 1.
Compounds were tentatively identified based on the measured protonated mass, fragmentation patterns, and isotopic ratios. Additionally, the identified VOCs were cross-referenced with the existing literature on spoilage compounds in meat (Senter et al. 2000; Lovestead and Bruno 2010; Alexandrakis et al. 2012). The limit of quantification for the spectra was set at ten standard deviations of the background noise registered for a blank sample (Franke and Beauchamp 2017). Proton transfer reaction rate constants used for quantitative analysis were based on the literature (Guthrie 1998; Zhao and Zhang 2004; Cappellin et al. 2012). When no data was available a rate of 2.0 × 10−9 cm3 s−1 was used.
Statistical Analysis
Data from the PTR-MS measurements was exported and further processed using a macro written in Visual Basic (Microsoft). Ten ions were then selected based on Pearson correlation coefficient (> 0.95) with results of TVB analysis over the period of 5 days. The chemometric analysis was performed using Orange v. 3.3.9 software (Demšar et al. 2013). In order to normalise the features, they were centred by mean and scaled by the standard deviation. Euclidean distances between the data points in a multidimensional space were then projected on a two-dimensional plane using multidimensional scaling (MDS) initiated with principal component analysis (PCA) and used as inputs for supervised pattern recognition. Support vector machines (SVM) method with RBF kernel was used to classify the data points into two groups (‘fresh’ and ‘suspect’) with 70% of data used for training and 30% for testing and with random sampling. The SVM technique is based on the separation of data embedded in a multidimensional plane with a hyperplane. The generalisation power with which the hyperplane separates the classes depends on the distance between the hyperplane and the closest data points which are called ‘support vectors’ (Cristianini and Scholkopf 2002; El Barbri et al. 2008).
Results and Discussion
Bacterial Growth
A typical growth curve of bacteria over a period of several days can be divided into three phases. First, there is a lag phase in which the organisms are getting used to the environment and their growth is limited. Afterwards, their population increases exponentially and then stabilises as an equilibrium between biomass production and cell death is reached. As shown in Fig. 2, the TVB value started to increase exponentially after the second day of cold storage which is consistent both with the literature (Jimenez et al. 1997; Mayr et al. 2003a) and with the shelf life declared by the manufacturers. Also shown in Fig. 2 is the corresponding concentration of acrolein determined in the same chicken breast sample. It should be noted that acrolein may be the product of not only bacterial metabolism, but also of chemical spoilage and has been shown to have a detrimental effect on the human health (Dadáková et al. 2012).
PTR-MS Analysis
The aim of this work was to compare the results of the bacteriological analysis with measured VOCs concentrations and to select compounds to be used as inputs for poultry freshness classification. The concentrations of volatile compounds averaged over the nine repetitions for a sample of chicken and turkey breast at day 1 and 5 are shown in Fig. 3. The overall concentrations of volatiles in the volatile fraction of the original samples increased significantly over the period of the experiment. This increase was, however, less noticeable in turkey meat, as compared to samples of chicken meat. The reason for that might be the fact that turkey meat is, in general, leaner than chicken meat, and so over time gives rise to fewer products of lipid oxidation (Chang and Watts 1952). Overall, the number of detected compounds for all the samples increased approximately sevenfold between day 1 and day 5 of storage.
Based on Pearson correlation test, ten different compounds detected in the headspace of sample 1a which best correlated with results of TBC analysis, performed on that same sample were then tentatively identified. It should be noted that a definitive identification of these compounds, listed in Table 1, in poultry’s volatile fraction might require the use of, e.g. gas chromatography coupled with mass spectrometry. The compounds were detected at a protonated mass (m + 1) due to their ionisation via a transfer of a proton from hydronium ions.
In order to verify whether it is possible to assess the freshness of poultry sourced from different retailers using concentration values of pre-determined compounds as input variables for statistical analysis, SVM was used for classification into two groups, namely ‘fresh’ and ‘suspect’, that is days 1–2 and 3–5 of refrigerated storage, respectively. A preliminary unsupervised analysis has shown that already at day 1 there are clear differences not only between chicken and turkey meat samples but also between samples of chicken meat from different sources. However, the differences between samples from the same supplier procured at two different dates were far less pronounced, as shown in Fig. 4. This suggests that the changes in the headspace composition of these samples in time might be determined not only by bacterial processes themselves and storage conditions but also by the initial composition of the sample, making a classification based on any pre-determined set of input variables more difficult. For that reason, only the changes in concentration level of the selected compounds in the headspace of samples 1a and 1b were listed in Table 1.
Using a supervised classification algorithm, it was possible to correctly classify 100% of replicates from the 1a sample (n = 45). This is not surprising as this is the one for which the TVC was run in parallel, and based on which the best-correlated input variables were determined. Using the same set of variables, it was also possible to correctly classify 100% of samples of chicken meat from the same source as the first one but procured 2 weeks later (sample 1b).
In chicken meat samples from different manufacturers and retailers (samples 2 and 3), the classification accuracy for the former was 93% with one false-positive and two false-negatives, while for the latter it was 100%. It should be noted that the occurrence of a false-positive result is a reason for more serious concern than a false-negative one, as it means that a sample which has exceeded its shelf life was classified as ‘fresh’ and might not be safe to consume. Finally, it was evaluated whether the compounds determined in a sample of chicken meat can be used as inputs in the freshness-based classification of meat from another species, namely turkey. In this case, the overall classification accuracy was 91%, however with three false-positives and one false-negative. The confusion matrices for the sample classification are depicted in Fig. 5.
The results suggest that it is possible to evaluate the shelf life of meat samples from the same source using a supervised data analysis technique based on the results of a prior analysis. With poultry from different manufacturers, the results are far less conclusive, as the initial bacterial flora of meat is very heterogenous in regard to number and composition. Additional variables might be introduced with slight differences in storage conditions as well as supply chain which makes the determination of a set of universal indicators of poultry spoilage unlikely. In order to draw more definite conclusions, it would be necessary to analyse a much larger number of different samples and to investigate the impact of storage conditions or even of the season on the outcome of classification. Furthermore, poultry meat is increasingly being distributed and stored in the MAP, and in such a scenario the proposed approach should be modified to include the use of modified atmosphere during refrigerated storage and as a carrier gas. The further stages of the development of a method for poultry shelf life assessment based on the use of PTR-TOFMS will involve continuous, real-time monitoring of the changes in the headspace of refrigerated poultry meat samples, and a complementary use of GC-MS for a more reliable identification of the volatiles. Additionally, in order to verify the robustness of the proposed approach a large number of samples will have to be analysed, including duck and goose meat stored in aerobic conditions and the results validated with more direct reference methods such as the determination of the biogenic amines index (Lázaro et al. 2015).
The shelf life assessment method proposed in this article should also, in principle, be applicable to other types of aerobically stored meat, such as pork, beef or mutton. Moreover, the proposed algorithm for multivariate analysis could be applied in rapid fingerprinting based on the results of analysis using other analytical techniques. For instance, instead of the concentration of selected ions measured using the PTR-MS, peak areas obtained using ultra-fast gas chromatography or response signals of chemical sensors used in electronic noses could be used as input data (Wojnowski et al. 2017c). Both gas chromatography and electronic olfaction are as of yet more readily available than PTR-MS and could find application in the evaluation of the shelf life of food products due to the relatively low cost of commercially available instruments.
Conclusions
It might be concluded that it is possible to evaluate rapidly the shelf life of poultry using PTR-MS based on the results of prior bacteriological analysis even when the classified sample was produced at a different time, provided that the meat came from the same source. This means that this technique could potentially be used to supplement the more traditional meat freshness evaluation methods such as TVC in facilities where meat is produced and distributed. The PTR-MS system has some major advantages over other techniques employed in the gas analysis. Its use allows for rapid, quantitative measurement without the need for extensive sample preparation and manipulation, at which point the bacterial flora of the sample might get upset. This way the risk of the occurrence of artefacts is minimised. Furthermore, the headspace concentrations can be determined without the use of standards, although qualitative analysis cannot be realised as reliably as with mass spectrometry techniques in which hard ionisation is used. Ideally, the rapid PTR-MS analysis could be combined with qualitative GC-based analyses of a handful of samples in order to identify the peaks and facilitate the interpretation of multidimensional data, and also to confirm the fragmentation pattern of the determined compounds.
The possibility of online monitoring of the time evolution of a meat sample’s headspace composition was not elaborated in this work; instead, the PTR-MS was used for fingerprinting as the potential for rapid and non-invasive assessment makes it a valuable tool for meat freshness evaluation. The time of a single analysis could be reduced to several seconds through the use of an automatic sampler. If the measurements were to be carried out in parallel with routine bacterial count analysis for an extended period of time the classification accuracy would increase with the number of measured samples. Indeed, if a large enough body of data regarding aromatic profiles correlated with the results of TVC analysis was gathered for artificial neural networks to be used it might even prove possible not only to classify but also to reliably predict the shelf life of poultry. Such a development would be especially advantageous as the results of the analysis would be available in a matter of minutes, as opposed to hours or even days with currently used methods. Due to the aforementioned advantages, the PTR-MS system might find application in instances where an automated, rapid method for monitoring of bacterial contamination of meat is required, for instance in butcheries, laboratories of institutions tasked with food safety assurance, or even large-scale retailers. The result would benefit not only the industry but also the consumer.
References
Alexandrakis D, Brunton NP, Downey G, Scannell AGM (2012) Identification of spoilage marker metabolites in Irish chicken breast muscle using HPLC, GC–MS coupled with SPME and traditional chemical techniques. Food Bioprocess Technol 5:1917–1923. https://doi.org/10.1007/s11947-010-0500-8
Amann A, Costello BDL, Miekisch W et al (2014) The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J Breath Res 8:34001. https://doi.org/10.1088/1752-7155/8/3/034001
Biasioli F, Gasperi F, Yeretzian C, Märk TD (2011) PTR-MS monitoring of VOCs and BVOCs in food science and technology. TrAC-Trends Anal Chem 30:968–977
Cappellin L, Karl T, Probst M, Ismailova O, Winkler PM, Soukoulis C, Aprea E, Märk TD, Gasperi F, Biasioli F (2012) On quantitative determination of volatile organic compound concentrations using proton transfer reaction time-of-flight mass spectrometry. Environ Sci Technol 46:2283–2290. https://doi.org/10.1021/es203985t
Chang ICL, Watts BM (1952) The fatty acid content of meat and poultry before and after cooking. J Am Oil Chem Soc 29:334–338. https://doi.org/10.1007/BF02639813
Cooksey K (2014) Modified atmosphere packaging of meat, Poultry and Fish. Elsevier Ltd
Cristianini N, Scholkopf B (2002) Support vector machines and kernel methods: the new generation of learning machines. AI Mag 23:31
Dadáková E, Pelikánová T, Kalač P (2012) Contents of biologically active polyamines in duck meat and giblets after slaughter and their changes during meat storage and cooking. Food Res Int 48:28–33. https://doi.org/10.1016/j.foodres.2012.02.016
Demšar J, Curk T, Erjavec A et al (2013) Orange: data mining toolbox in Python. J Mach Learn Res 14:2349–2353
El Barbri N, Llobet E, El Bari N et al (2008) Application of a portable electronic nose system to assess the freshness of Moroccan sardines. Mater Sci Eng C 28:666–670. https://doi.org/10.1016/j.msec.2007.10.056
Franke C, Beauchamp J (2017) Real-time detection of volatiles released during meat spoilage: a case study of modified atmosphere-packaged chicken breast fillets inoculated with Br. thermosphacta. Food Anal Methods 10:310–319. https://doi.org/10.1007/s12161-016-0585-4
Guthrie JP (1998) Predicting the rates of proton transfer reactions: a simple model using equilibrium constants and distortion energies. J Phys Org Chem 11:632–641. https://doi.org/10.1002/(SICI)1099-1395(199808/09)11:8/9<632::AID-POC47>3.0.CO;2-1
ISO 4833-2 (2013). Microbiology of the food chain—horizontal method for the enumeration of microorganisms -- Part 2: Colony count at 30 degrees C by the surface plating technique
Jeremiah L (2001) Packaging alternatives to deliver fresh meats using short- or long-term distribution. Food Res Int 34:749–772. https://doi.org/10.1016/S0963-9969(01)00096-5
Jimenez SM, Salsi MS, Tiburzi MC, Rafaghelli RC, Tessi MA, Coutaz VR (1997) Spoilage microflora in fresh chicken breast stored at 4 C: influence of packaging methods. J Appl Microbiol 83:613–618. https://doi.org/10.1046/j.1365-2672.1997.00276.x
Jordan A, Haidacher S, Hanel G, Hartungen E, Märk L, Seehauser H, Schottkowsky R, Sulzer P, Märk TD (2009) A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). Int J Mass Spectrom 286:122–128. https://doi.org/10.1016/j.ijms.2009.07.005
Lázaro CA, Conte-Júnior CA, Canto AC, Monteiro MLG, Costa-Lima B, Cruz AG, Mársico ET, Franco RM (2015) Biogenic amines as bacterial quality indicators in different poultry meat species. LWT-Food Sci Technol 60:15–21. https://doi.org/10.1016/j.lwt.2014.09.025
Lindinger W, Hansel A, Jordan A (1998) On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int J Mass Spectrom Ion Process 173:191–241. https://doi.org/10.1016/S0168-1176(97)00281-4
Lovestead TM, Bruno TJ (2010) Detection of poultry spoilage markers from headspace analysis with cryoadsorption on a short alumina PLOT column. Food Chem 121:1274–1282. https://doi.org/10.1016/j.foodchem.2010.01.044
Mayr D, Margesin R, Klingsbichel E et al (2003a) Detection of the spoiling of meat using PTR–MS. Int J Mass Spectrom 223:229–235. https://doi.org/10.1016/S1387-3806(02)00793-5
Mayr D, Margesin R, Klingsbichel E, Hartungen E, Jenewein D, Schinner F, Mark TD (2003b) Rapid detection of meat spoilage by measuring volatile organic compounds by using proton transfer reaction mass spectrometry. Appl Environ Microbiol 69:4697–4705. https://doi.org/10.1128/AEM.69.8.4697-4705.2003
Senter SD, Arnold JW, Chew V (2000) APC values and volatile compounds formed in commercially processed, raw chicken parts during storage at 4 and 13 C and under simulated temperature abuse conditions. J Sci Food Agric 80:1559–1564. https://doi.org/10.1002/1097-0010(200008)80:10<1559::AID-JSFA686>3.0.CO;2-8
Vul E, Harris C, Winkielman P, Pashler H (2009) Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci 4:274–290. https://doi.org/10.1111/j.1745-6924.2009.01125.x
Wojnowski W, Majchrzak T, Dymerski T, Gębicki J, Namieśnik J (2017a) Poultry meat freshness evaluation using electronic nose technology and ultra-fast gas chromatography. Monatshefte für Chemie - Chem Mon 148:1631–1637. https://doi.org/10.1007/s00706-017-1969-x
Wojnowski W, Majchrzak T, Dymerski T, Gębicki J, Namieśnik J (2017b) Electronic noses: powerful tools in meat quality assessment. Meat Sci 131:119–131. https://doi.org/10.1016/j.meatsci.2017.04.240
Wojnowski W, Majchrzak T, Dymerski T, Gębicki J, Namieśnik J (2017c) Portable electronic nose based on electrochemical sensors for food quality assessment. Sensors 17:2715. https://doi.org/10.3390/s17122715
Zhao J, Zhang R (2004) Proton transfer reaction rate constants between hydronium ion (H3O+) and volatile organic compounds. Atmos Environ 38:2177–2185. https://doi.org/10.1016/j.atmosenv.2004.01.019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Wojciech Wojnowski declares he has no conflict of interest. Tomasz Majchrzak declares that he has no conflict of interest. Piotr Szweda declares that he has no conflict of interest. Tomasz Dymerski declares that he has no conflict of interest. Jacek Gębicki declares that he has no conflict of interest. Jacek Namieśnik declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wojnowski, W., Majchrzak, T., Szweda, P. et al. Rapid Evaluation of Poultry Meat Shelf Life Using PTR-MS. Food Anal. Methods 11, 2085–2092 (2018). https://doi.org/10.1007/s12161-018-1193-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1193-2