Abstract
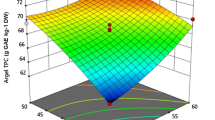
Psidium guajava L. has gained a special attention as health plant due to the presence of phenolic compounds. Box-Behnken design (BBD) has been applied for the extraction of target compounds from guava leaves via sonotrode ultrasound-assisted extraction (UAE). Different extraction times (5, 30, and 55 min), ratios of ethanol/water (50, 75, and 100% (v/v)), and ultrasound (US) power (80, 240, and 400 W) were tested to find their effect on the sum of phenolic compound (SPC), flavonols and flavan-3-ols via HPLC-ESI-QqQ-MS, and antioxidant activity (DPPH and TEAC assays). The best process conditions were as follows: 40 min, 60% ethanol/water (v/v), and 200 W. Established method has been used to extract phenolic compounds in two guava leaves varieties (pyrifera and pomifera). Pyrifera var. showed greater values of the SPC via HPLC-ESI-QqQ-MS (49.7 mg/g leaf dry weight (d.w.)), flavonols (12.51 mg/g d.w.), flavan-3-ols (7.20 mg/g d.w.), individual phenolic compounds, and antioxidant activity (8970 ± 5 and 465 ± 6 μmol Trolox/g leaf d.w, respectively) than pomifera var. Conventional extraction showed lower amounts of phenolic compounds (7.81 ± 0.03 and 4.64 ± 0.01 mg/g leaf d.w. for flavonols and flavan-3ols, respectively) in comparison to the ultrasound-assisted ones.


Similar content being viewed by others
References
Aybastier Ö, Işik E, Şahin S, Demir C (2013) Optimisation of ultrasonic-assisted extraction of antioxidant compounds from blackberry leaves using response surface methodology. Ind Crop Prod 44:558–565. doi:10.1016/j.foodchem.2013.04.003
Barba FJ, Galanakis CM, Esteve MJ et al (2015) Potential use of pulsed electric technologies and ultrasounds to improve the recovery of high-added value compounds from blackberries. J Food Eng 167:38–44. doi:10.1016/j.jfoodeng.2015.02.001
Barbalho SM, Farinazzi-Machado FMV, de Alvares GR et al (2012) Psidium guajava (guava): a plant of multipurpose medicinal applications. Med Aromat Plants 1:1–6. doi:10.4172/2167-0412.1000104
Baş D, Boyacı İH (2007) Modeling and optimization I: usability of response surface methodology. J Food Eng 78:836–845. doi:10.1016/j.jfoodeng.2005.11.024
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Leb Wiss u Technol 28:25–30
Chemat F, Zill-e-Huma KMK (2011) Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason Sonochem 18:813–835. doi:10.1016/j.ultsonch.2010.11.023
Deng Q, Zinoviadou KG, Galanakis CM et al (2014) The effects of conventional and non-conventional processing on Glucosinolates and its derived forms, Isothiocyanates: extraction, degradation, and applications. Food Eng Rev 7:357–381. doi:10.1007/s12393-014-9104-9
Dhaouadi K, Meliti W, Dallali S et al (2015) Commercial Lawsonia inermis L. dried leaves and processed powder: Phytochemical composition, antioxidant, antibacterial, and allelopathic activities. Ind Crop Prod 77:544–552. doi:10.1016/j.indcrop.2015.09.037
Díaz-de-Cerio E, Gómez-Caravaca AM, Verardo V et al (2016) Determination of guava (Psidium guajava L.) leaf phenolic compounds using HPLC-DAD-QTOF-MS. J Funct Foods 22:376–388. doi:10.1017/CBO9781107415324.004
Engström MT, Pälijärvi M, Salminen J-P (2015) Rapid fingerprint analysis of plant extracts for Ellagitannins, Gallic acid, and Quinic acid derivatives and quercetin-, Kaempferol- and myricetin-based flavonol glycosides by UPLC-QqQ-MS/MS. J Agric Food Chem 63:4068–4079. doi:10.1021/acs.jafc.5b00595
Ferreira SLC, Bruns RE, Ferreira HS et al (2007) Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597:179–186. doi:10.1016/j.aca.2007.07.011
Galanakis CM (2013) Emerging technologies for the production of nutraceuticals from agricultural by-products: a viewpoint of opportunities and challenges. Food Bioprod Process 91:575–579. doi:10.1016/j.fbp.2013.01.004
Galanakis CM (2012) Recovery of high added-value components from food wastes: conventional, emerging technologies and commercialized applications. Trends Food Sci Technol 26:68–87. doi:10.1016/j.tifs.2012.03.003
Galanakis CM, Schieber A (2014) Editorial. Food Res Int 65:299–300. doi:10.1016/j.foodres.2014.11.019
Gutiérrez RMP, Mitchell S, Solis RV (2008) Psidium guajava: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 117:1–27. doi:10.1016/j.jep.2008.01.025
Heng MY, Tan SN, Yong JWH, Ong ES (2013) Emerging green technologies for the chemical standardization of botanicals and herbal preparations. TrAC Trends Anal Chem 50:1–10. doi:10.1016/j.trac.2013.03.012
Hibbert DB (2012) Experimental design in chromatography: A tutorial review. J Chromatogr B 910:2–13. doi:10.1016/j.jchromb.2012.01.020
Jacotet-Navarro M, Rombaut N, Fabiano-Tixier A-S et al (2015) Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary. Ultrason Sonochem 27:102–109. doi:10.1016/j.ultsonch.2015.05.006
Kaur C, Kapoor HC (2001) Antioxidants in fruits and vegetables - the millennium’s health. Int J Food Sci Technol 36:703–725. doi:10.1046/j.1365-2621.2001.00513.x
Khan MK, Abert-Vian M, Fabiano-Tixier AS et al (2010) Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem 119:851–858. doi:10.1016/j.foodchem.2009.08.046
Liu C-W, Wang Y-C, Hsieh C-C et al (2015) Guava (Psidium guajava Linn.) leaf extract promotes glucose uptake and glycogen accumulation by modulating the insulin signaling pathway in high-glucose-induced insulin-resistant mouse FL83B cells. Process Biochem 50:1128–1135. doi:10.1016/j.procbio.2015.03.022
Mailoa MN, Mahendradatta M, Laga A, Djide N (2013) Tannin extract of guava leaves ( Psidium guajava L ) variation with concentration organic solvents. Int J Sci Technol Res 2:106–110
Morton JF (1987) Fruits of warm climates. Miami, FL
Pan Z, Qu W, Ma H et al (2012) Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason Sonochem 19:365–372. doi:10.1016/j.ultsonch.2011.05.015
Parejo I, Codina C, Petrakis C, Kefalas P (2000) Evaluation of scavenging activity assessed by Co ( II )/ EDTA-induced luminol chemiluminescence and DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical assay. J Pharmacol Toxicol Methods 44:507–512
Pingret D, Fabiano-Tixier AS, Le Bourvellec C et al (2012) Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. J Food Eng 111:73–81. doi:10.1016/j.jfoodeng.2012.01.026
Ramić M, Vidović S, Zeković Z et al (2015) Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason Sonochem 23:360–368. doi:10.1016/j.ultsonch.2014.10.002
Re R, Pellegrini N, Proteggente A et al (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Roselló-Soto E, Galanakis CM, Brnčić M et al (2015) Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci Technol 42:134–149. doi:10.1016/j.tifs.2015.01.002
Tao Y, Sun D-W (2015) Enhancement of food processes by ultrasound: a review. Crit Rev Food Sci Nutr 55:570–594. doi:10.1080/10408398.2012.667849
Tay P, Tan C, Abas F et al (2014) Assessment of extraction parameters on antioxidant capacity, polyphenol content, Epigallocatechin gallate (EGCG), Epicatechin gallate (ECG) and Iriflophenone 3-C-β-Glucoside of Agarwood (Aquilaria crassna) young leaves. Molecules 19:12304–12319. doi:10.3390/molecules190812304
Wang F, Guo XY, Zhang DN et al (2015) Ultrasound-assisted extraction and purification of taurine from the red algae Porphyra yezoensis. Ultrason Sonochem 24:36–42. doi:10.1016/j.ultsonch.2014.12.009
Wong BY, Tan CP, Ho CW (2013) Effect of solid-to-solvent ratio on phenolic content and antioxidant capacities of “Dukung Anak” (Phyllanthus niruri). Int Food Res J 20:325–330
Wong WH, Lee WX, Ramanan RN et al (2015) Two level half factorial design for the extraction of phenolics, flavonoids and antioxidants recovery from palm kernel by-product. Ind Crop Prod 63:238–248. doi:10.1016/j.indcrop.2014.09.049
Wong Paz JE, Muñiz Márquez DB, Martínez Ávila GCG et al (2014) Ultrasound-assisted extraction of polyphenols from native plants in the Mexican desert. Ultrason Sonochem 22:1–8. doi:10.1016/j.ultsonch.2014.06.001
Zeković Z, Vidović S, Vladić J et al (2014) Optimization of subcritical water extraction of antioxidants from Coriandrum sativum seeds by response surface methodology. J Supercrit Fluids 95:560–566. doi:10.1016/j.supflu.2014.09.004
Zhen J, Villani TS, Guo Y et al (2016) Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem 190:673–680. doi:10.1016/j.foodchem.2015.06.006
Zinoviadou KG, Galanakis CM, Brnčić M et al (2015) Fruit juice sonication: implications on food safety and physicochemical and nutritional properties. Food Res Int 77:743–752. doi:10.1016/j.foodres.2015.05.032
Acknowledgments
The author Elixabet Díaz-de-Cerio would like to thank to the University of Granada and the CEIBiotic for the “Convocatoria de movilidad internacional de jóvenes investigadores de programas de doctorado” grant. Vito Verardo thanks the Spanish Ministry of Economy and Competitiveness (MINECO) for “Juan de la Cierva” post-doctoral contract.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Not applicable.
Conflict of Interest
Elixabet Díaz-de-Cerio that she has no conflict of interest. Urszula Tylewicz that she has no conflict of interest. Vito Verardo that he has no conflict of interest. Alberto Fernández-Gutiérrez that he has no conflict of interest. Antonio Segura-Carretero that he has no conflict of interest. Santina Romani that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Informed Consent
Informed consent was not applicable.
Rights and permissions
About this article
Cite this article
Díaz-de-Cerio, E., Tylewicz, U., Verardo, V. et al. Design of Sonotrode Ultrasound-Assisted Extraction of Phenolic Compounds from Psidium guajava L. Leaves. Food Anal. Methods 10, 2781–2791 (2017). https://doi.org/10.1007/s12161-017-0836-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0836-z