Abstract
The aim of this work was to determine the level of contamination of different groups of vegetable oils available on the Polish market with polycyclic aromatic hydrocarbons, i.e. benzo(a)pyrene and sum of benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene, the content of which in foodstuffs is limited by Commission Regulation (EU) 2015/1125 of 10 July 2015. The research materials were refined rapeseed oils, sunflower oils, olive pomace oil, rapeseed oils with olive oil and unrefined soybean and coconut oils. The research methods included process of saponification of the vegetable oils, extraction of the polycyclic aromatic hydrocarbons fraction, clean-up by use a column packed with aluminum oxide and elution by petroleum ether, and then quantitative and qualitative determination of by high performance liquid chromatography with fluorescence detection method. Values of limit of detection and limit of quantification obtained during validation of the method were 0.18 and 0.25 μg/kg, respectively, and were significantly lower than the respective maximum values given in Commission Regulation (EU) 836/2011. The highest polyaromatic hydrocarbons content was found in unrefined coconut and soybean oils. The benzo(a)pyrene content and sum of benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene in all the tested sample did not exceed the maximum levels given in Commission Regulation (EU) 2015/1125.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vegetable fats are recognized as important components of the human diet. They are a source of energy and essential fatty acids as well as carriers of fat soluble vitamins. Fats are natural multi-mixture of different lipids, including triacylglycerols (97-99%) and accompanying substances in proportions depending on the type of raw material and methods of extraction and purification of the resulting product (Dubois et al. 2007; Zachara and Juszczak 2016). In addition to the unsaturated fatty acids content in oilseeds, vegetable oils and fatty products another important factor is the content of compounds with strong antioxidant properties, which large amounts determine the appropriate nutritional value and the storage stability of these products (Krygier et al. 2011). Foods high in fat are particularly susceptible to contamination with polycyclic aromatic hydrocarbons (PAHs). Due to lipophilic character they accumulate in the fat cells of plants and animals (Moret and Conte 2000; Wenzl et al. 2006). Vegetable oils may be contaminated with PAHs as a result of processing, especially drying of oil plants and due to use of contaminated extraction solvent. To a lesser extent a contamination of these oils may be associated with environmental pollution (Guillen et al. 2004; Lage Yusty and Cortizo Daviña, 2005; Moret et al. 2005; WHO 2010; Bojanowska and Czerwiński 2010); however, in a case of high air pollution with PAHs and due to atmospheric precipitation, a superficial contamination of plants during growing season may occur. This contamination may be then transferred to the final product (Rodríguez-Acuna et al. 2008). Both storage of seeds in silos and the processes of deodorization and cleaning affect the reduction of the level of contamination with PAHs. On the other hand, direct impact of exhaust gases during drying of the seeds and/or impact of high temperature during extraction of oil from seeds result in increased amount of polyaromatic hydrocarbons in a final product. Many researchers observed significant differences in the level of PAHs in different production batches of the same type of oil (Cejpek et al. 1998; Tys et al. 2003; Guillen et al. 2004).
Polycyclic aromatic hydrocarbons were evaluated by the International Programme on Chemical Safety (IPCS) of the World Health Organization (WHO) in 1998, the Scientific Committee on Food of the European Commission in 2002 and the Joint FAO/WHO Expert Committee on Food Additives in 2005. The International Agency for Research on Cancer has classified benzo(a)pyrene to the Group 1 of carcinogens, i.e. compounds with proven carcinogenic effect to humans, while benz(a)anthracene, benzo(b)fluoranthene and chrysene to Group 2B (compounds possibly carcinogenic to humans) (WHO 2010). The research results confirming the carcinogenic effect of PAHs on humans and the available data on food contamination with these compounds resulted in the adoption of the Commission Regulation (EC) 208/2005 of 4 February 2005 and then of the Commission Regulation (EC) 1881/2006 of 19 December 2006, in which maximum levels for benzo(a)pyrene in foodstuffs have been settled. Further regulations on maximum levels for polycyclic aromatic hydrocarbons in foodstuffs have been given in Commission Regulation (EU) 835/2011, where benzo(a)pyrene – previous marker for the occurrence of polycyclic aromatic hydrocarbons in food has been replaced with four specific substances: benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene. Moreover, maximum levels of sum of these four PAHs in different foodstuffs have been established. It was found that these compounds are present in foods in varying amounts, but their sum covers approx. 60% of fifteen carcinogenic PAHs listed in Commission Regulation (EU) 835/2011. According to the above mentioned Regulation, oils and fats (excluding cocoa butter and coconut oil) intended for direct human consumption or use as ingredients in food can contain maximum 2 μg/kg of benzo(a)pyrene, while sum of benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene is limited to the level of 10 μg/kg. Maximum level of sum of the four PAHs for coconut oil intended for direct human consumption or use as an ingredient in food is 20 μg/kg. This is due to proportionally higher benzo(a)pyrene and chrysene contents, which cannot be easily removed from coconut oil during refining process with the current technical possibilities of producing countries (Commission Regulation (EU) 2015/1125).
The PAHs in food are mainly determined with use of high performance liquid chromatography with fluorescence detection or gas chromatography-mass spectrometry (Sadowska-Rociek et al. 2015). The methods of liquid chromatography are more often used in food analysis due to the lower cost of equipment and lower cost of routine determination of the PAHs limited by law regulation. Moreover, they are sufficient to official food control. More expensive GC-MS technique is used in scientific works due to the possibility of determination and identification of a number of polycyclic aromatic hydrocarbons which affect food safety (Zachara and Juszczak 2016).
The European Union is the world’s largest producer of rape – it produces about 19-20 million tonnes per year, representing 34% of global production. Compared to other European Union countries, Poland ranks third in terms of production volume, after Germany and France (Dmochowska 2012). In Poland, rapeseed oil is the most popular fat and it is consumed by approx. 50% of consumers (Krygier et al. 2009). Much less people consume other types of vegetable oils: sunflower or soybean oils – approx. 4%, while olive oil – approx. 1.5% of consumers (Zachara and Juszczak 2016). In recent years, increased consumer interest in vegetable fats is observed and various types of rapeseed, sunflower, soybean and olive oils as well as their mixtures appeared on the market.
The current state of knowledge on genotoxic, mutagenic and carcinogenic properties of polycyclic aromatic hydrocarbons contributes to the fact that these compounds are of interest in a broad spectrum of sciences. According to the assessment of the European Commission it is necessary to constantly monitor the PAHs content in food, including fats of vegetable origin, for the continuous monitoring of risks.
The aim of this work was to determine, using validated chromatographic method, the level of contamination of different groups of vegetable oils available on the Polish market with polycyclic aromatic hydrocarbons, i.e. benzo(a)pyrene (BaP) and sum of benzo(a)pyrene (BaP), benz(a)anthracene (BaA), benzo(b)fluoranthene (BbFA) and chrysene (CHR), the content of which in foodstuffs is limited by Commission Regulation (EU) 2015/1125.
Materials and Methods
Determination of polycyclic aromatic hydrocarbons in the samples was performed by validated method that meets the criteria set out in the Commission Regulation (EU) 836/2011. The Laboratory is accredited in this analysis by Polish Accreditation Centre. The Laboratory participated in the inter-laboratory study conducted by the National Institute of Public Health – National Institute of Hygiene, which is the national reference laboratory and satisfactory results of determination of four PAHs in oil samples enriched with PAHs at two levels were obtained.
Materials and reagents
For the chromatographic analysis the following reagents were used: acetonitrile, cyclohexane, methanol (all of HPLC purity) (Merck, Darmstadt, Germany), sodium chloride and potassium hydroxide (all of analytical grade) (POCH, Gliwice, Poland). Water of HPLC purity was from LiChrosolv (Merck, Darmstadt, Germany), while nitrogen of analytical grade was from Air Liquide (Rzeszow, Poland). For the clean-up procedure, neutral aluminum oxide of activity stage I for column chromatography (Merck, Darmstadt, Germany), petroleum ether of HPLC purity (Merck, Darmstadt, Germany) and anhydrous sodium sulphite of analytical grade (POCH, Gliwice, Poland) were used. The certified standard PAH Solution Mix from AccuStandard (New Haven, USA) consisted of PAHs solution in methanol-dichloromethane (MeOH-DCM), with 200.6 μg/ml of BaP, 197.8 μg/ml of BaA, 198.8 μg/ml of BbFA and 199.0 μg/ml of CHR. In order to validate the method, certified reference material FAPAS 0618 Olive oil from Central Science Laboratory (York, UK) was used.
Apparatus
HPLC was used for the determination and quantitation of each of the PAHs. The UltiMate 3000 (Dionex, Sunnyvale, U.S.A.) chromatographic system was used, which consisted of WPS-3000TSL auto-sampler, DGP-3600A pump, TCC-3200 2×2P-10P thermostated column compartment, RF 2000 fluorescence detector, connected to Chromeleon software (version 6.80 SP2 Build 2284). The Hypersil Green PAH (tailored alkyl-bonded silica with high carbon loading; Thermo Scientific, Waltham, U.S.A.) column (250×4.6 mm, I.D., 5 μm) and guard column (10×4.0 mm, I.D., 5 μm) were used.
Samples
Samples of edible oils: 30 samples of rapeseed oil, 10 samples of sunflower oil and 12 samples of other oils: olive pomace oil, rapeseed oil with olive oil, soybean oil and coconut oil were commercially available. Samples of rapeseed oil included universal refined oils, virgin rapeseed oils and virgin cold-filtered refined rapeseed oil and they were both of domestic origin (12 samples) and imported (18 samples). Before analysis the samples were stored according to the manufacturer’s recommendations. Liquid samples were conditioned at room temperature within 2 hrs, while solid samples were melted in a water bath at 60 °C (PN-EN ISO 661:2006).
Analytical procedure
Isolation of the hydrocarbon fraction
In order to get PAH extracts clean enough for chromatographic analysis the purification step with use of glass chromatographic column packed with alumina was performed. Mixture of aluminum oxide and water (9:1, w/w) was poured into a glass column equipped with fritted glass and the column was tapped to pack it. A anhydrous sodium sulphite as a drying agent was made on the alumina packing. The column was conditioning by passing petroleum ether.
A 0.4 g aliquot of oil was diluted with 10 ml of petroleum ether and the sample was loaded onto the alumina column. Then, 60 ml of petroleum ether was poured onto the column with flow rate for approx. 1 ml/min. The first 20 ml eluted fraction was discarded. The exact fraction (PAH compounds) was collected. The solution was evaporated in Laborota 4000 rotary evaporator (Heidolph, Schwabach, Germany) at 35 °C under vacuum to the final volume of less than 1 ml and then the sample was quantitatively transferred to vials and evaporated under a gentle stream of nitrogen.
In the case of coconut oil samples the liquid-liquid extraction was preceded by a saponification step. For this purpose, a 2 g aliquot of sample was hydrolyzed with 1.5 M potassium hydroxide in methanol for 90 min at reflux. The hydrolyzed sample was then filtered, extracted three times with 50 ml of cyclohexane and washed three times with 50 ml of water. The resulting extract was dried by addition of anhydrous sodium sulphate and the supernatant was concentrated in a vacuum evaporator at 50 °C. The concentrated solution was quantitatively transferred to the 25 ml flask and made up to volume with petroleum ether. The resulting sample was purified on the alumina column according to the above described procedure. Each sample was prepared in duplicate.
HPLC- FLD analysis
An aliquot of 100 μl was injected into HPLC using an auto-sampler. The temperature of the column was maintained constant at 18 °C. The mobile phase was constituted of acetonitrile and water. The elution conditions applied were: 0 – 3 min, 60% of acetonitrile isocratic; 3 – 15 min, 60-100% of acetonitrile gradient, 15 – 46 min, 100% of acetonitrile isocratic, 46 – 53 min, 100-60% of acetonitrile, gradient. The flow rate was 1.0 ml/min. The effluents were monitored using the following excitation and emission (Ex/Em) wavelengths: 260/420 nm for BaA and CHR and 290/430 nm for BbFA and BaP.
Preparation of calibration curve
Calibration curves were performed by external standard method. The working standard solution at a concentration of 10 ng/ml was made with the certified standard solution of PAHs and it was used to make the following calibration solutions: 0.20, 0.80, 4.00, 8.00 and 10.00 ng/ml. The calibration curves were plotted as linear dependence (y = a∙x) of the measured signal (y) in function of the peak area of the standard substance (x).
Measurement of samples
The measurement procedure consisted of dosing the following samples: blind samples, oil samples, two calibration solutions (1.00 and 9.00 ng/ml) and oil samples enriched with certified standard sample or with certified reference material.
Statistical analysis
Determination of the working range and linearity of the method
For each of PAH calibration curves the coefficients of variation for concentration limits were calculated and then the F-Snedecor test was used for testing homogeneity of coefficients of variation at a 0.05 significance level. Correlation coefficient (r) was also calculated. The limit of detection (LOD) and the limit of quantification (LOQ) were calculated according to the following formulas: C m+3SD and C m+6SD, respectively, where C m is mean concentration of PAH in a sample of very low PAH concentration and SD is standard deviation. The sensitivity of the method was measured as the slope of the calibration curve.
Statistical analysis of results
Results obtained in the procedure of validation of the method were tested by Dixon Q test for identification and rejection of outliers. Then, for each of PAH concentrations the following statistical parameters were calculated: recovery factor (correctness), variance, standard deviation, coefficient of variation, standard and expanded uncertainties, confidence interval and relative standard deviation of repeatability. The evaluation of the significance of differences between mean values of PAHs was made using Tukey multiple comparison test at a 0.05 significance level. All statistical procedures were computed using Statistica version 10.0 (StatSoft, Poland).
Results and discussion
The values of the limit of detection (LOD), the limit of quantification (LOQ) and the recovery complied with the criteria set out in the Commission Regulation (EU) 836/2011 (Table 1). In the concentration range of 0.20-10.00 ng/ml the calibration curves for each of the standard substances were linear, with values of correlation coefficient higher than 0.998. These curves were used to check the reproducibility and precision of the method at two concentration levels of 0.80 and 8.00 ng/ml. Acceptable results were obtained, therefore this part of the study allowed for approval to use the method for determining the sum of four PAHs in vegetable oils.
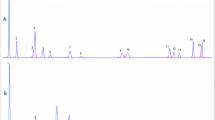
Examples of chromatogram profiles of the determined PAHs in standard solution and in refined rapeseed oils are presented in Fig. 1 and Fig. 2, respectively.
The determined limited benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene as well as sum of these four PAHs contents of rapeseed oils are given in Table 2. The PAHs concentration values were corrected for the recoveries obtained for each of the measurement series, in accordance with the requirements of the Commission Regulation (EU) 836/2011. The recovery values were within the ranges of 80-103%, 87-109%, 87-108% and 91-110% for benzo(a)pyrene, benz(a)anthracene, chrysene and benzo(b)fluoranthene, respectively.
The refined rapeseed oils contained benzo(a)pyrene in the amount not exceeding the permissible limit of 2.0 μg/kg (Commission Regulation (EU) 2015/1125). In two samples of the refined rapeseed oils and in one sample of refined virgin rapeseed oil the BaP content was higher than one (1.20 ± 0.29 μg/kg, 1.92 ± 0.29 μg/kg and 1.10 ± 0.17 μg/kg, respectively).
Maximum amount of the analyzed PAHs in the rapeseed oil samples ranged from 1.30 to 2.40 μg/kg, while in the refined virgin rapeseed oil samples it was from 1.10 to 1.95 μg/kg. Taking into account the value of uncertainty this is not a significant difference. Significantly lower amounts of benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene, being lower than 1 μg/kg, were found in the cold-filtered refined virgin rapeseed oil samples (Table 2). For these samples, also the sum of four PAHs (2.91 ± 0.58 μg/kg) was significantly lower than in the rapeseed oils and in refined virgin rapeseed oils.
Chrysene was the most quantitatively dominant compound of all the PAHs determined in the rapeseed oils, with its content exceeding 1 μg/kg in three samples of the rapeseed oils and in four samples of the refined virgin rapeseed oils. This phenomenon is in agreement with that of Alomirah et al. (2010), who determined chrysene in 83% of vegetable oil samples, while benz(a)anthracene in 70% of them.
The sum of four PAHs in all the samples did not exceed the permissible value of 10 μg/kg of product (Table 2). Our results of determination of PAHs in refined rapeseed oils, with relatively the highest chrysene content, are in agreement with these reported by Ciecierska and Obiedziński (2006). While, Węgrzyn et al. (2006) determined lower PAHs content in refined rapeseed oils (BaP – 0.24 μg/kg; Σ4PAHs – 1.49 μg/kg) than in the present study. These authors also found significant differences in benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene contents between refined rapeseed oils, cold-filtered refined virgin rapeseed oils and rapeseeds. According to Ciecierska and Obiedziński (2006) refining process play a key role in the oilseeds processing, because it significantly reduces polyaromatic hydrocarbons content in the final product. However, the amounts of benzo(a)pyrene determined by Jędra et al. (2008) in refined rapeseed oils (3.74 μg/kg) and in refined virgin rapeseed oils (0.92-1.03 μg/kg) were significantly higher than, and similar to, respectively, these obtained in the present work. The levels of contamination of rapeseed oils with benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene were similar to the average levels of these compounds found in refined vegetable oils tested in the EU countries (EFSA 2008).
On an industrial scale the oil from oilseeds is usually obtained in two stages: first by hot mechanical pressing in screw presses, and then by extracting the residual oil from pomace. Oil is often subjected to a refining process in order to remove some impurities such as metal ions, pesticides and polycyclic aromatic hydrocarbons (PAHs). In the literature there is a lack of data on levels of contamination of cold filtered extra virgin rapeseed oils with polycyclic aromatic hydrocarbons. The analysed rapeseed oils were advertised as 100% refined virgin rapeseed oils, cold filtered; however, according to the producer information given on the labels they were cold filtered at the bottling stage. Filtration plays an important role in the purification technology of vegetable oils. One of the most popular filtration methods used for rapeseed oils is filtration on vertical plate filters at 50°C. After pressing, the oil contains a significant amount of pomace, a by-product of oil manufacture. Pressed oil is of better quality than the extracted one (Krygier et al. 2009, 2011). Starski and Jędra (2011) reported that there were differences in PAHs level of rapeseed oils produced by different manufacturers and they concluded that it could be due to regionalization of rape crops and differences in filtration methods.
In the analysed refined sunflower oils the benzo(a)pyrene content did not exceed the level of 2.0 μg/kg (Table 3). In 30% of the samples the BaP content was lower than LOD (0.18 μg/kg) determined during method validation (Table 1), while 50% of the samples contained from 0.18 to 0.25 μg/kg of BaP (Table 3). In most refined sunflower oil samples the BaP, CHR and BbFA content was lower than LOQ (in 80%, 70% and 80%, respectively). Similar results were reported by other authors (Teixeira et al. 2007; Węgrzyn et al. 2006; Alomirah et al. 2010; Dost and Ideli 2012). The highest PAHs contents (1.86 ± 0.28 μg/kg; 0.92 ± 0.14 μg/kg; 1.42 ± 0.21 μg/kg and 1.58 ± 0.24 μg/kg for BaP, BaA, CHR and BbFA, respectively) were found in one sample of refined sunflower oil originating from UE (Table 3); however, in this case the sum of four PAHs (5.78 ± 1.16 μg/kg) and BaP content did not exceed the permissible limits given in Commission Regulation (EU) 2015/1125. The levels of contamination of the refined vegetable oils with PAHs are comparable to these found by other authors (Cejpek 1998; Guillen et al. 2004; Teixeira et al. 2007). Most authors (Ciecierska and Obiedziński 2006; Starski and Jędra 2011) reported that the PAHs content of edible oils was reduced as a result of refining process, especially by bleaching with use of a mixture of adsorbents (activated bleaching earth and activated charcoal) and by deodorisation, while Teixeira et al. (2007) observed a slight increase in PAHs content of soybean oil and olive oil after the bleaching process.
None of the tested samples of popular Polish olive pomace oil and rapeseed oil with 5% of olive oil exceeded the maximum permissible PAHs levels and their minimum benzo(a)pyrene content was below the limit of detection, while the maximum BaP content amounted to 0.25 and 0.45 μg/kg for olive pomace oil and rapeseed oil with olive oil, respectively (Table 4). This result is consistent with this one reported by Jędra et al. (2008), where BaP content of olive pomace oil amounted to 0.31 μg/kg. No statistically significant difference was found between the maximum sums of four polycyclic aromatic hydrocarbons determined in olive pomace oil (3.15 ± 0.63 μg/kg) and rapeseed oil with olive oil (2.92 ± 0.58 μg/kg). Rodríguez-Acuña and Pérez-Caminomdel (2008) found that the PAHs content of olive oil and of olives is dependent on environmental pollution and exposition of the fruits to fumes. Polycyclic aromatic hydrocarbons are adsorbed on the surface of the fruit and can be transferred to the oil during extraction process. According to Ciecierska and Obiedziński (2006) olive pomace oil can be a significant food source of PAHs. These authors determined 61 μg/kg of benzo(a)pyrene and more than 96 μg/kg of sum of the four PAHs in this product, with the first value exceeding 30 times the permissible limit. High amount of polycyclic aromatic hydrocarbons in olive pomace oil results from direct drying process of the pomace before oil extraction (Ciecierska and Obiedziński 2006). Guillen et al. (2004) and Wu and Yu (2012) found that the PAHs content can be a useful factor in determining quality of different vegetable oils and in optimizing refining process.
Benzo(a)pyrene and sum of four PAHs contents of unrefined soybean coconut oils were significantly higher than these of refined oils (Table 4). The amounts of each of the determined PAHs in unrefined soybean oils were higher than 0.7 μg/kg, while maximum sum of four PAHs amounted to 9.10 ± 1.82 μg/kg. The latter did not exceed the permissible level for vegetable oils (Commission Regulation (EU) 2015/1125). Significantly lower PAHs contents were determined by Yu et al. (2014) in soybean and crude and refined soybean oils. These authors found that benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene contents were reduced as a result of neutralization, bleaching and deodorization of the oils.
The highest contamination with polycyclic aromatic hydrocarbons was found for unrefined coconut oils (Table 4); however, due to much higher limits for the sum of the four PAHs established for coconut oil used for direct human consumption or as an ingredient in foods (20 μg/kg) the determined sum of four PAHs do not exceed the permissible level. Coconut oil is usually obtained by pressing a dry coconut meat yielding crude protein oil, which is then filtered, washed and refined.
In the present study, a significant linear correlation (r = 0.9470) between benzo(a)pyrene content and sum of four PAHs was found.
Our results of determination of the four limited polycyclic aromatic hydrocarbons in different edible vegetable oils are consistent with these presented in the EFSA report published in 2008 and involving 2100 vegetable oil samples originating from 17 Member States, where 85.8% of the samples contained less than 2 μg/kg of benzo(a)pyrene. Moreover, also chrysene was the most quantitatively dominant PAH of all the determined polycyclic aromatic hydrocarbons (EFSA 2008).
Conclusions
All the tested vegetable oil samples showed a benzo(a)pyrene (BaP) and sum of benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene (sum of four PAHs) levels below established limit values given in Commission Regulation (EU) 2015/1125. The unrefined oils were characterized by significantly higher contamination with PAHs as compared to the refined oils. The unrefined coconut oil contained the highest level of PAHs. No significant differences were found between the four PAHs contents of the refined rapeseed oils and the refined virgin rapeseed oils, while the maximum levels of each of the PAHs determined in the cold-filtered refined virgin rapeseed oils were significantly lower than these found in other rapeseed oils.
References
Alomirah H, Al-Zenki S, Husain A, Sawaya W, Ahmed N, Gevao B, Kannan K (2010) Benzo[a]pyrene and total polycyclic aromatic hydrocarbons (PAHs) levels in vegetable oils and fats do not reflect the occurrence of the eight genotoxic PAHs. Food Addit Contam 27(6):869–878. doi:10.1080/19440040903493793
Bojanowska M, Czerwiński J (2010) Polycylic aromatic hydrocarbons in rape seeds with relation to their growing site and thermal treatment. J Toxicol Env Heal A 73(17,8):1250–1259. doi:10.1080/15287394.2010.492013
Cejpek K, Hajslova J, Kocourek V, Tomaniova M, Cmolik J (1998) Changes in PAH levels during production of rapeseed oil. Food Addit Contam 15(5):563–574. doi:10.1080/02652039809374682
Ciecierska M, Obiedziński M (2006) Vegetable oils contamination by polycyclic aromatic hydrocarbons. Food. Science. Technology. Quality 2(47):48–55
Commission Regulation (EC) No 208/2005 of 4 February 2005 amending Regulation (EC) No 466/2001 as regards polycyclic aromatic hydrocarbons. Official Journal of the European Union L 34, 8.2.2005.
Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuff. Official Journal of the European Union L 364, 20.12.2006.
Commission Regulation (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Official Journal of the European Union L 215, 20.8.2011
Commission Regulation (EU) No 836/2011 of 19 August 2011 amending Regulation (EC) No 333/2007 laying down the methods of sampling and analysis for the official control of the levels of lead, cadmium, mercury, inorganic tin, 3-MCPD and benzo(a)pyrene in foodstuffs. Official Journal of the European Union L 215, 20.8.2011
Commission Regulation (EU) 2015/1125 of 10 July 2015 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in Katsuobushi (dried bonito) and certain smoked Baltic herring. Official Journal of the European Union L 184, 11.7.2015
Dmochowska H (ed) (2012) International statistics yearbook. Central Statistical Office of Poland, Warsaw
Dost K, Ideli C (2012) Determination of polycyclic aromatic hydrocarbons in edible oils and barbecued food by HPLC/UV–Vis detection. Food Chem 133:193–199. doi:10.1016/j.foodchem.2012.01.001
Dubois V, Breton S, Linder M, Fanni J, Parmentier M (2007) Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur J Lipid Sci Tech 109:710–732. doi:10.1002/ejlt.200700040
EFSA (2008) A report from the unit of data collection and exposure on a request from the European Commission Findings of the EFSA data collection on polycyclic aromatic hydrocarbons in food. First issued on 29 June 2007 and revised on 31 July 2008. Available on: http://www.efsa.europa.eu/en/scdocs/doc/33r.pdf (November 2015)
Guillen M, Sopelana P, Palencia G (2004) Polycyclic aromatic hydrocarbons and olive pomace oil. J Agr Food Chem 52:2123–2132. doi:10.1021/jf035259q
Jędra M, Starski A, Gawarska H, Sawilska-Rautenstrauch D (2008) Benzo(a)pyrene contamination of vegetable oils. Rocz Panstw Zakl Hig 59(2):131–138
Krygier K, Wroniak M, Ptasznik S (2009) The impact of the different stages of production of rapeseed oil for its health benefits. In: Krzymański J (ed) Rapeseed oil—the new raw material, new truth. PSPO-Publishing, Warsaw, pp. 31–43
Krygier K, Wroniak M, Maszewska M (2011) The impact of technological processes on the nutritional value of edible oils. In: Nowak D (ed) Food quality and safety. Shaping the nutritional quality in industrial processes. SGGW-Publishing, Warsaw, pp. 65–75
Lage Yusty MA, Cortizo Daviña JL (2005) Supercritical fluid extraction and high-performance liquid chromatography-fluorescence detection method for PAHs investigation in vegetable oils. Food Control 16:59–64. doi:10.1016/j.foodcont.2003.11.008
Moret S, Conte LS (2000) Polycyclic aromatic hydrocarbons in edible fats and oils: occurrence and analytical methods. Journal of Chromatogr A 882:245–253. doi:10.1016/S0021-9673(00)00079-0
Moret S, Purcaro G, Conte LS (2005) Polycyclic aromatic hydrocarbons in vegetable oils from canned foods. Eur J Lipid Sci Tech 107:488–496. doi:10.1002/ejlt.200501060
PN-EN ISO 661 (2006) Animal and vegetable fats and oils. Preparation of test sample
Rodríguez-Acuna R, Pérez-Caminomdel C, Cert A, Moreda W (2008) Sources of contamination by polycyclic aromatic hydrocarbons in Spanish virgin olive oils. Food Addit Contam A 25:115–122. doi:10.1080/02652030701459855
Sadowska-Rociek A, Surma M, Cieślik E (2015) Determination of polycyclic aromatic hydrocarbons in coffee and coffee substitutes using dispersive SPE and gas chromatography-mass spectrometry. Food Anal Methods 8:109–121. doi:10.1007/s12161-014-9876-9
Starski A, Jędra M (2011) Assessment of polycyclic aromatic hydrocarbons level in rapeseed oils manufactured in Poland. Bromatology and Chemical Toxicology XLIV 4:1054–1060
Teixeira VH, Casal S, Oliveira MBPP (2007) PAHs content in sunflower, soybean and virgin olive oils: evaluation in commercial samples and during refining process. Food Chem 104:106–112. doi:10.1016/j.foodchem.2006.11.007
Tys J, Rybacki R, Malczyk P (2003) Rapeseed contamination with benzo(a)pyrene in the north part of Poland. Oilseed Crops 34:627–636
Węgrzyn E, Grześkiewicz S, Popławska W, Głód BK (2006) Modified analytical method for polycyclic aromatic hydrocarbons, using SEC for sample preparation and RP-HPLC with fluorescence detection. Application to different food samples. Acta Chromatogr 17:233–249
Wenzl T, Simon R, Klieiner J, Anklam E (2006) Analytical methods for polycyclic aromatic hydrocarbons (PAHs) in food and the environment needed for new food legislation in the European Union. Trend Anal Chem 25:716–725. doi:10.1016/j.trac.2006.05.010
World Health Organization, International Agency for Research on Cancer (2010) IARC monographs on the evaluation of carcinogenic risks to humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Lyon
Wu S, Yu W (2012) Liquid–liquid extraction of polycyclic aromatic hydrocarbons in four different edible oils from China. Food Chem 134:597–601. doi:10.1016/j.foodchem.2012.02.155
Yu Y, Qingzhe J, Wang Y, Gu Y, Wang X (2014) Sources of polycyclic aromatic hydrocarbons in soybean oil and its dynamic changes refining processing. Adv J Food Sci Technol 6(1):42–47
Zachara A, Juszczak L (2016) Food contamination by polycyclic aromatic hydrocarbons—legal requirements and monitoring. Food Science Technology Quality 3(106):5–20
Acknowledgments
The authors thank Monika Hoły for technical assistance in carrying out the analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alicja Zachara declares that she has no conflict of interest. Dorota Gałkowska declares that she has no conflict of interest. Lesław Juszczak declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zachara, A., Gałkowska, D. & Juszczak, L. Method Validation and Determination of Polycyclic Aromatic Hydrocarbons in Vegetable Oils by HPLC-FLD. Food Anal. Methods 10, 1078–1086 (2017). https://doi.org/10.1007/s12161-016-0673-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0673-5