Abstract
Background
Understanding the pathways by which interventions achieve behavioral change is important for optimizing intervention strategies.
Purpose
We examined mediators of behavior change in a tailored-risk communication intervention that increased guideline-based colorectal cancer screening among individuals at increased familial risk.
Methods
Participants at increased familial risk for colorectal cancer (N = 481) were randomized to one of two arms: (1) a remote, tailored-risk communication intervention (Tele-Cancer Risk Assessment and Evaluation (TeleCARE)) or (2) a mailed educational brochure intervention.
Results
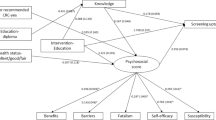
Structural equation modeling showed that participants in TeleCARE were more likely to get a colonoscopy. The effect was partially mediated through perceived threat (β = 0.12, p < 0.05), efficacy beliefs (β = 0.12, p < 0.05), emotions (β = 0.22, p < 0.001), and behavioral intentions (β = 0.24, p < 0.001). Model fit was very good: comparative fit index = 0.95, root-mean-square error of approximation = 0.05, and standardized root-mean-square residual = 0.08.
Conclusion
Evaluating mediating variables between an intervention (TeleCARE) and a primary outcome (colonoscopy) contributes to our understanding of underlying mechanisms that lead to health behavior change, thus leading to better informed and designed future interventions.
Trial Registration Number
ClinicalTrials.gov, NCT01274143.



Similar content being viewed by others
References
Society AC. Cancer Facts and Figures 2016, 2016.
Butterworth AS, Higgins JP, Pharoah P: Relative and absolute risk of colorectal cancer for individuals with a family history: A meta-analysis. Eur J Cancer. 2006, 42:216–227.
Johns LE, Houlston RS: A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001, 96:2992–3003.
Samadder NJ, Cannon-Albright LA, Burt RW: The impact of family history on the risk of colorectal neoplasia: Don’t change the guidelines just yet! Dig Dis Sci. 2012, 57:3047–3049.
National Comprehensive Cancer Network I: NCCN Practice Guidelines in Oncology, Colorectal Cancer Screening. Jenkintown, PA, 2007.
Levin B, Lieberman DA, McFarland B, et al.: Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008, 134:1570–1595.
Winawer S, Fletcher R, Rex D, et al.: Colorectal cancer screening and surveillance: Clinical guidelines and rationale—Update based on new evidence. Gastroenterology. 2003, 124:544–560.
Courtney RJ, Paul CL, Carey ML, et al.: A population-based cross-sectional study of colorectal cancer screening practices of first-degree relatives of colorectal cancer patients. BMC Cancer. 2013, 13:13.
Rubinstein WS, Acheson LS, O’Neill SM, et al.: Clinical utility of family history for cancer screening and referral in primary care: A report from the Family Healthware Impact Trial. Genet Med. 2011, 13:956–965.
Ruthotto F, Papendorf F, Wegener G, et al.: Participation in screening colonoscopy in first-degree relatives from patients with colorectal cancer. Ann Oncol. 2007, 18:1518–1522.
Honein-AbouHaidar GN, Kastner M, Vuong V, et al. Systematic review and meta-study synthesis of qualitative studies evaluating facilitators and barriers to participation in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2016.
Burt R, Winawer S, Bond J, Levin B, Sandler R. Preventing colorectal cancer: A clinician’s guide: American Gastroenterological Association., 2004.
Tyler CV, Jr., Snyder CW: Cancer risk assessment: Examining the family physician’s role. J Am Board Fam Med. 2006, 19:468–477.
Jones RM, Woolf SH, Cunningham TD, et al.: The relative importance of patient-reported barriers to colorectal cancer screening. Am J Prev Med. 2010, 38:499–507.
Anderson AE, Henry KA, Samadder NJ, Merrill RM, Kinney AY: Rural vs urban residence affects risk-appropriate colorectal cancer screening. Clin Gastroenterol Hepatol. 2013, 11:526–533.
Denberg TD, Melhado TV, Coombes JM, et al.: Predictors of nonadherence to screening colonoscopy. J Gen Intern Med. 2005, 20:989–995.
Steinwachs D, Allen JD, Barlow WE, et al.: National Institutes of Health state-of-the-science conference statement: Enhancing use and quality of colorectal cancer screening. Ann Intern Med. 2010, 152:663–667.
Kinney AY, Boonyasiriwat W, Walters ST, et al.: Telehealth personalized cancer risk communication to motivate colonoscopy in relatives of patients with colorectal cancer: The family CARE randomized controlled trial. J Clin Oncol. 2014, 32:654–662.
Steffen LE, Boucher KM, Damron BH, et al.: Efficacy of a telehealth intervention on colonoscopy uptake when cost is a barrier: The family CARE cluster randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2015, 24:1311–1318.
Jensen JD, King AJ, Carcioppolo N, Davis L: Why are tailored messages more effective? A multiple mediation analysis of a breast cancer screening intervention. J Commun. 2012, 62:851–868.
Kreuter MW, Wray RJ: Tailored and targeted health communication: Strategies for enhancing information relevance. Am J Health Behav. 2003, 27 Suppl 3:S227–232.
Lustria ML, Noar SM, Cortese J, et al.: A meta-analysis of web-delivered tailored health behavior change interventions. J Health Commun. 2013, 18:1039–1069.
Noar SM, Benac CN, Harris MS: Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull. 2007, 133:673–693.
Glasgow RE, Marcus AC, Bull SS, Wilson KM: Disseminating effective cancer screening interventions. Cancer. 2004, 101:1239–1250.
Pengchit W, Walters ST, Simmons RG, et al.: Motivation-based intervention to promote colonoscopy screening: An integration of a fear management model and motivational interviewing. J Health Psychol. 2011, 16:1187–1197.
Witte K: Putting the fear back into fear appeals: The extended parallel process model. Communication Monographs. 1992, 59:329–349.
Witte K: Fear control and danger control: A test of the extended parallel process model (EPPM). Communication Monographs. 1994, 61:113–134.
Witte K, Meyer G, Martell D: Effective Health Risk Messages: A Step-by-Step Guide, Thousand Oaks, CA: Sage Publications, Inc, 2001.
Gollwitzer P: Implementation intentions—Strong effects of simple plans. American Psychologist. 1999, 54:493–503.
Kwasnicka D, Presseau J, White M, Sniehotta F: Does planning how to cope with anticipated barriers facilitate health-related behaviour change? A systematic review. Health Psychology Review. 2013, 7:129–145.
Schwarzer R, Lippke S, Ziegelmann J: Health action process approach—A research agenda at the Freie Universitat Berlin to examine and promote health behavior change. Zeitschrift Fur Gesundheitspsychologie. 2008, 16:157–160.
Sheeran P: Intention-behavior relations: A conceptual and empirical review. European Review of Social Psychology. 2002, 12:1–36.
Gollwitzer P, Sheeran P: Implementation intentions and goal achievement: A meta-analysis of effects and processes. Advances in Experimental Social Psychology. 2006, 38:69–119.
Schweiger Gallo I, Gollwitzer PM: Implementation intentions: Control of fear despite cognitive load. Psicothema. 2007, 19:280–285.
Sheeran P, Orbell S: Using implementation intentions to increase attendance for cervical cancer screening. Health Psychol. 2000, 19:283–289.
Sheeran P, Silverman M: Evaluation of three interventions to promote workplace health and safety: Evidence for the utility of implementation intentions. Soc Sci Med. 2003, 56:2153–2163.
O’Connor AM, Jacobsen MJ, Stacey D: An evidence-based approach to managing women’s decisional conflict. J Obstet Gynecol Neonatal Nurs. 2002, 31:570–581.
Greiner KA, Daley CM, Epp A, et al.: Implementation intentions and colorectal screening: A randomized trial in safety-net clinics. Am J Prev Med. 2014, 47:703–714.
Miller MW, Rollnick S: Motivational Interviewing : Helping People Change (3rd Ed.). New York: Guilford Press, 2013.
Hall K, Gibbie T, Lubman DI: Motivational interviewing techniques—Facilitating behaviour change in the general practice setting. Aust Fam Physician. 2012, 41:660–667.
Hall K, Staiger PK, Simpson A, Best D, Lubman DI: After 30 years of dissemination, have we achieved sustained practice change in motivational interviewing? Addiction. 2016, 111:1144–1150.
Miller WR, Rollnick S: The effectiveness and ineffectiveness of complex behavioral interventions: Impact of treatment fidelity. Contemp Clin Trials. 2014, 37:234–241.
Kreuter MW, Chheda SG, Bull FC: How does physician advice influence patient behavior? Evidence for a priming effect. Arch Fam Med. 2000, 9:426–433.
Madlensky L, Esplen MJ, Gallinger S, McLaughlin JR, Goel V: Relatives of colorectal cancer patients: Factors associated with screening behavior. Am J Prev Med. 2003, 25:187–194.
Bastani R, Glenn BA, Taylor VM, et al.: Integrating theory into community interventions to reduce liver cancer disparities: The Health Behavior Framework. Prev Med. 2010, 50:63–67.
Rothman AJ: “Is there nothing more practical than a good theory?”: Why innovations and advances in health behavior change will arise if interventions are used to test and refine theory. Int J Behav Nutr Phys Act. 2004, 1:11.
Glanz K, Bishop DB: The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010, 31:399–418.
Tan YY, McGaughran J, Ferguson K, et al.: Improving identification of lynch syndrome patients: A comparison of research data with clinical records. Int J Cancer. 2013, 132:2876–2883.
Boonyasiriwat W, Hung M, Hon SD, et al.: Intention to undergo colonoscopy screening among relatives of colorectal cancer cases: A theory-based model. Ann Behav Med. 2014, 47:280–291.
Family CARE (Colorectal Cancer Awareness and Risk Education) Project (FCARE). Retrieved June 7, 2016, from http://rtips.cancer.gov/rtips/programDetails.do?programId=24393369.
Simmons RG, Lee YC, Stroup AM, et al.: Examining the challenges of family recruitment to behavioral intervention trials: Factors associated with participation and enrollment in a multi-state colonoscopy intervention trial. Trials. 2013, 14:116.
Birmingham WC, Hung M, Boonyasiriwat W, et al.. Effectiveness of the extended parallel process model in promoting colorectal cancer screening. Psychooncology. 2015.
Mack LA, Cook LS, Temple WJ, et al.: Colorectal cancer screening among first-degree relatives of colorectal cancer patients: Benefits and barriers. Ann Surg Oncol. 2009, 16:2092–2100.
Manne S, Markowitz A, Winawer S, et al.: Understanding intention to undergo colonoscopy among intermediate-risk siblings of colorectal cancer patients: A test of a mediational model. Prev Med. 2003, 36:71–84.
Gili M, Roca M, Ferrer V, Obrador A, Cabeza E: Psychosocial factors associated with the adherence to a colorectal cancer screening program. Cancer Detect Prev. 2006, 30:354–360.
Manne S, Markowitz A, Winawer S, et al.: Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early onset colorectal cancer. Health Psychol. 2002, 21:3–15.
Sifri R, Rosenthal M, Hyslop T, et al.: Factors associated with colorectal cancer screening decision stage. Prev Med. 2010, 51:329–331.
Greiner KA, Engelman KK, Hall MA, Ellerbeck EF: Barriers to colorectal cancer screening in rural primary care. Prev Med. 2004, 38:269–275.
Klabunde CN, Schenck AP, Davis WW: Barriers to colorectal cancer screening among Medicare consumers. Am J Prev Med. 2006, 30:313–319.
Shokar NK, Carlson CA, Shokar GS: Physician and patient influences on the rate of colorectal cancer screening in a primary care clinic. J Cancer Educ. 2006, 21:84–88.
Cheah W, Zimmerman R: Self-Construal and Fear Appeals: An Empirical Examination of College Students’ Gonorrhea Risk Perceptions. International Communication Association. New York, 2005.
Gurmankin Levy A, Shea J, Williams SV, Quistberg A, Armstrong K: Measuring perceptions of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006, 15:1893–1898.
Rawl SM, Menon U, Champion VL, et al.: Do benefits and barriers differ by stage of adoption for colorectal cancer screening? Health Educ Res. 2005, 20:137–148.
Weinrich SP, Weinrich MC, Boyd MD, Johnson E, Frank-Stromborg M: Knowledge of colorectal cancer among older persons. Cancer Nurs. 1992, 15:322–330.
McCaul K, Goetz P. Worry. Health Behavior Constructs: Theory measurement, and Research. Retrieved June 1, 2016 from http://cancercontrol.cancer.gov/brp/.
Horowitz M, Wilner N, Alvarez W: Impact of Event Scale: A measure of subjective stress. Psychosom Med. 1979, 41:209–218.
Hay J, Primavera L, Levy A, Shuk E, Ostroff J: Development and validation of a scale assessing novel cancer-related risk perceptions. Ann Behav Med. 2006, 31:S190.
Hay J, Shuk E, Cruz G, Ostroff J: Thinking through cancer risk: Characterizing smokers’ process of risk determination. Qual Health Res. 2005, 15:1074–1085.
Fritz MS, Mackinnon DP: Required sample size to detect the mediated effect. Psychol Sci. 2007, 18:233–239.
MacCallum R, Browne M, Sugawara H: Power analysis and determination of sample size for covariance structure modeling. Psychological Methods. 1996, 1:130–149.
Thoemmes F, Mackinnon DP, Reiser MR: Power analysis for complex mediational designs using Monte Carlo methods. Struct Equ Modeling. 2010, 17:510–534.
Wolf EJ, Harrington KM, Clark SL, Miller MW: Sample size requirements for structural equation models: An evaluation of power, bias, and solution propriety. Educ Psychol Meas. 2013, 76:913–934.
Hooper D, Coughlan J, Mullen MR: Structural equation modelling: Guidelines for determining model fit. Electronic Journal of Business Research Methods. 2008, 6:53–60.
Savalei V, Falk CF: Robust two-stage approach outperforms robust full information maximum likelihood with incomplete nonnormal data. Struct Equ Modeling. 2014, 21:280–302.
Graham JW: Missing data analysis: Making it work in the real world. Annu Rev Psychol. 2009, 60:549–576.
Ryan P: Integrated theory of health behavior change: Background and intervention development. Clin Nurse Spec. 2009, 23:161–170; quiz 171-162.
Laiyemo AO, Adebogun AO, Doubeni CA, et al.: Influence of provider discussion and specific recommendation on colorectal cancer screening uptake among U.S. adults. Prev Med. 2014, 67:1–5.
Blase K, Fixsen D. Core intervention components: Identifying and operationalizing what makes programs work: U.S. Department of Health & Human Services, 2013.
Rabin B, Glasgow RE: An implementation science perspective on psychological science and cancer: What is known and opportunities for research, policy, and practice. Am Psychol. 2015, 70:211–220.
Almario CV, May FP, Ponce NA, Spiegel BM: Racial and ethnic disparities in colonoscopic examination of individuals with a family history of colorectal cancer. Clin Gastroenterol Hepatol. 2015, 13:1487–1495.
Bromley EG, May FP, Federer L, Spiegel BM, van Oijen MG: Explaining persistent under-use of colonoscopic cancer screening in African Americans: A systematic review. Prev Med. 2015, 71:40–48.
McCarthy AM, Bristol M, Domchek SM, et al.. Health care segregation, physician recommendation, and racial disparities in BRCA1/2 testing among women with breast cancer. J Clin Oncol. 2016.
Gil A, Wagner E, Vega W: Acculturation, familism, and alcohol use among Latino adolescent males: Longitudinal relations. Journal of Community Psychology. 2000, 28:443–458.
Acknowledgements
We would like to thank Marc Schwartz, PhD; Antoinette Stroup, PhD; Lisa Pappas, MStat; Rebecca Simmons, PhD, MPH; and Randall Burt, MD for their contributions to the study design and execution. We also thank the interventionists who are genetic counselors in High Risk Clinical Research at Huntsman Cancer Center: Wendy Kohlmann, MS; Amanda Gammon, MS; Kory Jasperson, MS; Anne Naumer, MS; and Lisa Wadge, MS. We thank A.J. Figueredo, PhD, for consulting on the statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This manuscript included Family Colorectal Cancer Awareness and Risk Education (Family CARE) Project data obtained from the Kinney Research Group and is registered on the ClinicalTrials.gov website (NCT01274143). Family CARE was funded by the National Cancer Institute (1R01CA125194-0305; Kinney, PI) and the Huntsman Cancer Foundation. Family CARE was also supported by the Shared Resources (P30 CA042014) at Huntsman Cancer Institute; the Utah Cancer Registry, which is funded by Contract No. HHSN261201000026C from the National Cancer Institute’s SEER Program with additional support from the Utah State Department of Health and the University of Utah; the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885, the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01PC-2010-00034C awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N01-PC-54404 awarded to the Public Health Institute, and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58CCU000807-05 awarded to the Public Health Institute; the Colorado Central Cancer Registry program in the Colorado Department of Public Health and Environment funded by the National Program of Cancer Registries of the Centers for Disease control and Prevention; the Cancer Data Registry of Idaho supported in part by the National Program of Cancer Registries of the Centers for Disease Control and prevention; the University of New Mexico Comprehensive Cancer Center Support Grant: Development Funds and the Biostatistics Shared Resource (P30CA118100; C.L.W.); the New Mexico Tumor Registry which is funded by National Cancer Institute Contract No. HHSN261201000033C; the Rocky Mountain Cancer Genetics Network (HHSN261200744000C); the Huntsman Cancer Registry; the University of Utah Department of Orthopaedics and the Center for Outcomes Research and Assessment; and the Intermountain Healthcare Oncology Clinical Program and Intermountain Clinical Genetic Institute. This content is solely the responsibility of the authors and does not necessarily reflect the opinions or views of the funding and supporting agencies.
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards
Authors Barbara H. Brumbach, Wendy C. Birmingham, Watcharaporn Boonyasiriwat, Scott Walters, and Anita Y. Kinney declare that they have no conflict of interest. All procedures, including the informed consent process, were approved by the Institutional Review Boards of participating institutions and were conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000.
Electronic Supplementary Material
.
ESM 1
(DOCX 14 kb)
About this article
Cite this article
Brumbach, B.H., Birmingham, W.C., Boonyasiriwat, W. et al. Intervention Mediators in a Randomized Controlled Trial to Increase Colonoscopy Uptake Among Individuals at Increased Risk of Familial Colorectal Cancer. ann. behav. med. 51, 694–706 (2017). https://doi.org/10.1007/s12160-017-9893-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12160-017-9893-1