Abstract
This study investigated the impact of process configuration and conditions on microbial communities and metabolic pathways in the anaerobic digestion of winery effluents. Four system configurations were analyzed for taxonomic and functional profiles using 16S rRNA gene sequencing and Tax4Fun2. Sporolactobacillus, Prevotella, and Acetobacter dominated (> 70%) in the acidogenic reactor with 5277 conserved functions across configurations. In the methanogenic reactor, methane production relied on Methanosaeta in the single-stage configuration (13%) and five archaea genera in the two-stage configuration (18%). Thermophilic conditions favored syntrophic acetate oxidation and hydrogenotrophic methanogenesis by Methanothermobacter (65%), significantly changing due to temperature. The two-stage configuration exhibited 3.0 times higher functional redundancy than the single-stage configuration. Mesophilic conditions displayed 2.5 times greater functional redundancy than thermophilic conditions. High organic loading rate and short hydraulic retention time reduced functional redundancy by 1.5 times. Assessing microbial functionality beyond their composition is crucial to understand stability and performance of anaerobic digestion systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agro-industrial wastes have attracted increasing attention during the last decade because they can be used as raw materials in biotechnological processes, producing energy sources, chemical products, and biomaterials [1, 2]. Some of these promising raw materials are winery effluents or winery wastewaters, produced during winemaking and characterized by high concentrations of chemical oxygen demand (> 200 g COD L−1), total solids (> 50 g TS L−1), and ethanol (> 100 g COD L−1), among other compounds [3]. Anaerobic digestion is an attractive option for treating winery effluents. Methane [4] and other products, such as biohydrogen [5] or medium-chain carboxylic acids [6], can also be obtained. Recently, highly concentrated winery effluents were successfully treated to produce methane in single-stage and two-stage (acidogenic and methanogenic) reactors, obtaining better performance in the two-stage configuration and reaching higher productivity in thermophilic than in mesophilic conditions [3, 7].
Typically, changes in the performance of anaerobic processes are attributed to the process configuration, environment, and operating conditions [8]. However, microbial communities, structure, dynamics, and metabolism deserve further exploration to understand the strategies behind improving methane production or modifying the process stability [9, 10]. Currently, molecular tools aid in determining the structure and composition of anaerobic microbial communities, known as the taxonomic profile [11]. Moreover, several tools have been developed to predict functional metabolic pathways and key functional genes based on 16S rDNA data, referred to as the functional profile [12]. Among these tools, PICRUSt and Tax4Fun have been used to study anaerobic digestion processes [13, 14].
Most studies on anaerobic digestion primarily rely on taxonomic profiles to describe and infer microbial community functions. However, it has been demonstrated that the taxonomic profile alone does not guarantee accurate inferences about functions [15]. Furthermore, functional profiles exhibit greater conservation and resilience across microbial communities compared to taxa [16]. On the other hand, studies that incorporate both taxonomic and functional profiles in laboratory experiments often focus on investigating the impact of a single variable on the system [13, 17]. This narrow approach hinders the ability to compare and extrapolate the simultaneous effects of multiple factors. As a result, there is a lack of studies that effectively combine taxonomic and functional approaches while considering their connection with process configuration, performance, and factors influencing process stability within the same system. It is essential to bridge this gap in research to obtain a more comprehensive and accurate understanding of anaerobic systems.
For all the above, this work aimed to compare the taxonomic and functional profiles of four process configurations of an anaerobic digestion system treating winery effluents. Microbial communities were identified using 16S rDNA marker genes, and metabolic functions were predicted using Tax4Fun2, a second-generation tool that includes the prediction of functional gene redundancy. The analysis of these profiles explains, for the first time, the effect of different process configurations, such as separated stages (acidogenic–methanogenic), temperature conditions, and changes in operating parameters, such as organic loading rate (OLR) and hydraulic retention time (HRT), over microbial communities and metabolic pathways related to methane production and process stability in the same system.
Materials and Methods
Anaerobic Digestion System
The anaerobic digestion system consisted of two reactors, the acidogenic reactor (A) and the methanogenic reactor (M), operating under four distinct operational conditions. These conditions involved mesophilic (35 °C) or thermophilic (55 °C) temperatures, as indicated in Table 1. The acidogenic reactor (A) was a continuous stirred tank reactor (CSTR) with a total volume of 1 L (0.6 L of working volume). On the other hand, the methanogenic reactor (M) was a CSTR with a total volume of 7 L (4 L of working volume). Condition 1 involved a single-stage methanogenic reactor (M1) operating at a mesophilic temperature. Condition 2 consisted of a two-stage process (A2-M2), both operating under mesophilic conditions. Condition 3 was also a two-stage process (A3-M3), similar to condition 2, but with the acidogenic reactor operating at mesophilic temperature and the methanogenic reactor operating at thermophilic temperature. Lastly, condition 4 replicated the configuration and temperatures of condition 3, but with an increased OLR for both reactors (A4-M4) (Table 1). The start-up conditions and the acidogenic and methanogenic performances were previously reported [3, 7]. The single-stage system (M1) and the acidogenic reactors (A2, A3, and A4) were fed with untreated winery effluents. The effluent of the acidogenic reactors was fed to the methanogenic reactors in the two-stage configurations (M2, M3, and M4). The acidogenic reactors were subsequently operated from configurations 2 to 4, i.e., the biomass was not replaced when changing from one configuration to another. The methanogenic reactors of configurations 1 and 2 were subsequently operated; then, for configuration 3, the biomass was replaced by an inoculum acclimated to thermophilic conditions; configurations 3 and 4 were subsequently operated. Regarding the comparison among methanogenic reactors, conditions 1 and 2 aimed to compare single- and two-stage processes. Configuration 3 was compared with configuration 2 to determine the effect of thermophilic conditions in methanogenesis. Furthermore, configuration 4 was compared with configuration 3 to determine the effect of harsher operating conditions (HOC), i.e., high-OLR and short-HRT.
Sampling, DNA Extraction, and Sequencing
Samples of 20 mL were taken from each reactor at the end of each operating condition (1 to 4). It is essential to mention that the end of each operating condition was defined considering the stable operation of each reactor. The criterion for acidogenic reactors was a coefficient of variation of less than 10% in the COD of the acidogenic effluent. A coefficient of variation of less than 10% in methane production was considered for methanogenic reactors [3, 7]. Samples were immediately stored at − 20 °C. DNA was extracted from 0.25 g of concentrated biomass of every sample, using the DNeasy PowerMax Soil Kit (Qiagen, Netherlands) and quantified by spectrophotometry using a NANODrop 2000c (Thermo Scientific, USA). DNA samples were submitted to the Research and Testing Laboratory (RTL, Lubbock, USA) for Illumina MiSeq sequencing analysis of bacteria and archaea communities (16S rRNA). For samples of the acidogenic reactors, primers for the V6–V8 regions of the 16S gene (B969F = ACGCGHNRAACCTTACC—BA1406R = ACGGGCRGTGWGTRCAA) were employed, targeting bacteria [18]. For samples of the methanogenic reactors, primers for the V4 region of the 16S gene (515FB = GTGYCAGCMGCCGCGGTAA—806RB = GGACTACNVGGGTWTCTAAT) were employed, targeting archaea and bacteria [19].
Data Analysis
For the taxonomic profiles, the sequences were analyzed using the software package “DADA2” v1.6 in the R environment [20]. Briefly, to keep high sequences’ quality score (Q > 30), forward and reverse sequences’ length was truncated at 280 and 220 nucleotides, for bacteria, and at 270 and 200 nucleotides, for bacteria and archaea, respectively. Pairing, assembly, and chimera removal were performed following the DADA2 pipeline suggestions. Sequences with more than two expected errors were discarded, and the chimeric sequences were removed with the removeBimeraDenovo function, method = “consensus.” On average, more than 70,000 sequences were recovered from each sample, and the nonchimeric/merged sequenced ratio was 0.64 ± 0.1. Taxonomic classification was assigned using the SILVA release v138 database. Alpha diversity indices, including Chao1, Shannon, and Simpson, were calculated to compare the richness and diversity among samples using the phyloseq package in R. Most dominant genera were taxonomically related to the information of the global MiDAS4 database (Microbial Database for Activated Sludge) and other literature reports, to obtain an indicator on their possible function in the process [21]. The community distance caused by different temperature digestions was determined by the dissimilarity index Bray–Curtis and permutational multivariate analysis of variance (PERMANOVA), using the vegan package. To determine microbial taxa with significantly different abundance among operation temperature, the differential gene expression analysis was conducted based on the negative binomial distribution (DeSeq2), using the microbiomeMarker package, with a p-value cutoff of 0.05 to detect differences.
For the prediction of functional profiles, the software package Tax4Fun2 was used in the R environment [22]. Briefly, Tax4Fun2 uses the taxonomic profile to predict the metabolic pathways in the Kyoto Encyclopedia of Genes and Genomes (KEGG). The package assigns the ASVs (amplicon sequence variants) to reference sequences in the SILVA database (release v132), and the taxonomic abundance is transformed to the relative abundance of enzymes encoding genes in the KEGG pathways and orthologs. The functional redundancy (FR) was calculated for each sample employing the functional redundancy index (FRI) of the Tax4Fun2 package. The FRI refers to the proportion of community members capable of possessing a particular KEGG function and their phylogenetic relationship. For example, a log ratio (FRIA/FRIB) greater than 0 indicates that a function is more redundant in A. Conversely, a log ratio below 0 indicates that a function is more redundant in B [22].
Results and Discussion
Taxonomic Profile of the Acidogenic Reactors
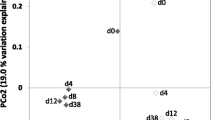
Bacterial communities were identified in the acidogenic reactors during configurations 2 to 4 (A2, A3, and A4). The bacterial community mainly comprised the phylum Firmicutes in A2, Bacteroidota in A3, and Proteobacteria in A4 (Fig. S1a). The taxonomic profile at the genus level (Fig. 1) shows that the predominant genera changed among the configurations. However, the most abundant genus displayed a relative abundance higher than 70% in all three cases. The genus Sporolactobacillus, predominant in A2, was previously reported in acidogenic reactors devoted to H2 production, treating highly concentrated substrates, such as molasses wastewater and winery effluents [23, 24]. In such systems, this genus was related to the production of lactic acid and volatile fatty acids (VFA). The genus Prevotella, predominant in A3, was reported with relative abundances between 14 and 57.5% in acidogenic reactors treating food waste and sugar refinery wastewater and was related to VFA production, including acetic and butyric acids [25, 26]. The genus Acetobacter, predominant in A4, was reported in acidogenic reactors treating tequila vinasses [27]. Acetobacter produces acetic acid from sugars and ethanol-rich environments under anaerobic conditions, so it is commonly found in wine bottles [28], explaining its presence in the treatment of winery effluents. The second most important genus in the acidogenic reactor was Lactobacillus, found in all three configurations. This genus was identified in the fermentation of several substrates, including tequila vinasses [27], molasses [29], and cheese whey [30], and it is commonly found in the acidogenic stage of two-stage anaerobic digestion systems treating food waste [31]. In acidogenic reactors, the presence of Lactobacillus is related to the production of lactic acid from carbohydrates before other genera consume the lactic acid to produce VFA [30]. The alpha diversity indexes of community richness (Chao1) and community diversity (Shannon and Simpson) were calculated for the acidogenic reactors (Fig. S1b). The results of these indices indicate a loss of richness and microbial diversity among the acidogenic reactors, which were subsequently operated from conditions 2 to 4.
It was found that the structure of the microbial community changed substantially from configuration 2 to 4, along with a loss of community richness and diversity. However, it should be noted that despite environmental changes, the acidogenic reactors consistently produced acetate as the primary fermentation product in all three configurations (Table 1). The main environmental changes in this reactor were related to differences in the OLR and variations in the influent composition. Most studies have found that variations in OLR and pH drive significant changes in the structure of microbial communities of acidogenic reactors. However, despite these community changes, acidogenic reactors can produce a consistent profile of fermentation products [32]. The loss of richness and diversity in the acidogenic reactors could be beneficial, as Yin et al. recently reported for 42 sludges from acidogenic lab-scale reactors [33]. Those authors suggested that the presence of some critical microorganisms and metabolic functions and not diversity should be considered when optimizing the acidogenic process. As noted in this taxonomic profile analysis, significant changes in the structure of microbial communities do not provide sufficient information to understand the response of the process to environmental changes, so it is necessary to analyze the functional profile of these communities.
Metabolic Pathways and Functional Redundancy in the Acidogenic Reactors
The functional analysis comprises metabolic pathway categories from the KEGG database distributed in three levels. At the shallowest level (level 3; Fig. S2a), no difference was observed in the acidogenic reactors between configurations 2, 3, and 4. Also, no difference was observed at level 2 in the metabolism category (Fig. S2b). Meanwhile, Fig. 2 shows the most profound level (level 1) categories for carbohydrate metabolism and energy metabolism, where only slight differences were found. In carbohydrate metabolism, categories such as starch and sucrose metabolism, galactose metabolism, and amino sugar and nucleotide sugar metabolism predicted a slightly higher relative abundance for A2 and A3 than for A4. The main difference in energy metabolism was a higher relative abundance in oxidative phosphorylation A4 > A3 > A2, probably related to Acetobacter, the most abundant genus in A4, and its ability to grow under aerobic conditions [28]. The present study shows that the abundance of the metabolic pathways predicted for the acidogenic reactors was very similar, despite the significant differences in the structure of the microbial community during reactor operation. These results indicate that the microbial community’s functional profile is decoupled from the taxonomic profile, which is better known as FR. This concept means that various taxonomically different species share similar or identical functions and can be replaced by one another to maintain the reactor’s functionality [34]. Other studies have reported that FR is an essential feature in acidogenic reactors [35]. The FR is directly related to the degree of stability and resilience to disturbances in the microbial community [36].
a Relative abundances of metabolic pathways on KEGG categories of carbohydrate metabolism (level 1). b Relative abundances of metabolic pathways on KEGG categories of energy metabolism (level 1). c Venn diagram showing unique and shared predicted functions in the acidogenic reactors among process configurations
The Venn diagram in Fig. 2c shows that the microbial community within the acidogenic reactors shared up to 5277 metabolic functions during its operation in the three configurations. Most of these functions are related to the metabolism category. Figure 2c also shows that unique metabolic functions were gradually lost in the acidogenic reactors, going from 348 in A2 to 82 in A3 and only 9 in A4. Since the acidogenic reactors were subsequently operated, this analysis suggests a specialization process of the metabolic functions; most of the unique functions in the community were lost in favor of conserving the shared metabolic functions that allowed the acidogenic process. The FR of these 5277 shared functions was compared among configurations. The FRI results showed that more functions had higher FR in A2 compared with A3, A2 compared with A4, and A3 compared with A4 (Fig. S3). The reduction of the FR in the acidogenic reactors indicates that functional genes existed in fewer species with a closer phylogenetic relationship at the end of the operation. In general, it was found that in the acidogenic reactors, a loss of diversity was accompanied by metabolic specialization and a reduction in FR. This finding could indicate the potential destabilization and reduced microbial community resilience. However, the relationship between diversity and FR in the acidogenic process deserves further study due to insufficient information on this topic and the evidence in recent reports [33].
Taxonomic Profile of the Methanogenic Reactors
Bacterial and archaeal communities were identified in the methanogenic reactors during configurations 1 to 4 (M1, M2, M3, and M4). The most abundant phyla were Firmicutes, Synergistota, Bacteroidota, and Proteobacteria for bacteria and Halobacterota, and Euryarhaeota for archaea (Fig. S4a). The taxonomic profile, including bacteria and archaea, shows essential differences in the community structure among configurations (Fig. 3). These differences are discussed in the following subsections, comparing single-stage vs. two-stage methanogenic reactors (M1 vs. M2), mesophilic vs. thermophilic conditions (M2 vs. M3), and changes in operating parameters, from low-OLR and long-HRT to high-OLR and short-HRT (M3 vs. M4).
Taxonomic Profile M1 vs. M2
The taxonomic profiles of the methanogenic reactors in configurations 1 and 2 were compared. It was found that bacteria dominated the microbial community in M1, with functions possibly related to the formation of precursors for producing methane, such as acetate or lactate from Lactobacillus and Citrobacter [37], or H2 and CO2 from bacteria possibly belonging to the group of syntrophic acetate-oxidizing bacteria (SAOB), such as Syner-01 [38]. In the single-stage reactor (M1), Methanosaeta dominated the archaeal fraction. That archaeon is commonly found in anaerobic digesters performing acetoclastic methanogenesis [21]. On the other side, it was found that the microbial community in M2 was remarkably more diverse than in M1 (Fig. S4b). The function of the most abundant genera in M2 may be fermentative metabolism, which is directed toward protein fermentation, related to the bacteria genera Proteiniphilum and Thermovirga [21]. In reactor M2, a diverse community of archaea was responsible for methane production. Acetoclastic methanogenesis could be attributed to Methanosaeta, while hydrogenotrophic methanogenesis could be attributed to Methanobacterium, Methanocorpusculum, Methanolinea, and Methanoculleus [21]. According to the diversity indexes (Fig. S4b), M2 had higher microbial richness and diversity than M1. Several studies have compared microbial communities in single- and two-stage configurations. Most studies evidence a higher diversity of bacteria and archaea in the two-stage configurations [39]. Other studies found no differences [40], and some studies reported higher diversity in the single-stage configuration, but they were made under thermophilic conditions [41]. The results of the present study suggest that the two-stage configuration promotes microbial diversity in the methanogenic reactor, allowing the growth of microorganisms with a greater diversity of metabolisms, which may explain the superior stability and resilience of the process compared to the single-stage configuration [3].
Taxonomic Profile M2 vs. M3
The differences between the taxonomic profile of configurations 2 and 3 (M2 vs. M3) could explain the differences between the process performance in mesophilic and thermophilic conditions. It was found that the archaeal community was more abundant under thermophilic conditions (65%) than under mesophilic conditions (20%). This difference in community structure could explain the higher yields obtained under thermophilic conditions, which were closer to the maximum theoretical yield (Table 1). Another possible explanation could be related to the efficiency of the methane production mechanism between temperature conditions. Under mesophilic conditions, the predominant mechanism in M2 could be a mixture of acetoclastic and hydrogenotrophic methanogenesis carried out by several archaea genera. The microbial community in M3 was composed of Methanothermobacter, a thermophilic hydrogenotrophic methanogen; Syntrophaceticus, classified as SAOB; and Acetomicrobium, which is a fermentative producer of acetate, hydrogen, and CO2 [21]. Therefore, under thermophilic conditions, the predominant mechanism in M3 could be the syntrophic acetate oxidation (SAO) coupled to hydrogenotrophic methanogenesis, carried out by a single predominant genus of archaea. Regardless of the higher yields, it has been reported that the stability of anaerobic digestion is affected under thermophilic conditions, which is directly related to the microbial community [9]. According to the diversity indexes (Fig. S4b), M2 had higher microbial richness and diversity than M3. Several studies have compared and reported a higher diversity of microbial communities in mesophilic than in thermophilic conditions using substrates such as food waste and sewage sludge [42, 43]. The operational temperature caused a community change, corroborated by the significant ecological distance among them (Fig. S5) and the clear difference in community composition (Fig. 3). The differential analysis showed that the Methanothermobacter genera significantly changed due to thermophilic temperature (log fold change = 14.1, p-value = 0.0001). This work confirmed the differences between community structures and the higher diversity in mesophilic conditions, which could provide greater resilience and stability to the mesophilic process.
Taxonomic Profile M3 vs. M4
Figure 3 shows that changing to HOC in the methanogenic reactor (high-OLR, short-HRT) affected the taxonomic profile even when the duration was only seven days. For example, the relative abundance of Methanothermobacter increased by 8.1%, and some genera of bacteria disappeared, such as Defluviitoga, capable of producing acetate, H2, and CO2 as fermentation products. In addition, and according to the diversity indexes, the richness and diversity of the community were significantly reduced (Fig. S4b). The reported effect of raising the OLR and reducing the HRT is the loss of communities with particular metabolic functions [44]. In the thermophilic methanogenic reactor, bacteria capable of using acetate and other substrates were lost after operation in HOC. The first effect of this change in operating conditions was a significant increase in methane production. However, after this increase in productivity, it was reported that the process suffered instability and overloading, gradually reducing the process efficiency [7]. Therefore, it can be inferred that applying HOC affects the resilience and stability of the process through the loss of microbial communities.
Functional Profile of the Methanogenic Reactors
According to the KEGG database categories, the metabolic pathways and functions were predicted for the methanogenic reactors in all configurations (M1, M2, M3, and M4). Some of the main differences were related to the energy metabolism category (level 1; Fig. S6a). The relative abundance of predicted functions related to methane metabolism was twice as high in the configurations under thermophilic conditions (M3 and M4) compared to those under mesophilic conditions (M1 and M2). This result can be attributed to the higher proportion of archaea in the thermophilic configurations. Other slight differences in metabolism were observed in the categories of sulfur metabolism and carbon fixation pathways in prokaryotes. Some other meaningful differences were found in specific functions of the general categories of cellular community, cell motility, membrane transport, and signal transduction (level 1; Fig. S6b). The main differences showed a higher abundance in M2 of metabolic pathways related to quorum sensing, biofilm formation, and metabolite transport, among others. These results could indicate that biofilm formation is favored in the two-stage mesophilic methanogenic reactor. However, the level of detail of this first functional approach does not allow for relating the taxonomic profile with the functions or mechanisms proposed for each abundant genus of microorganisms.
For this reason, a more detailed analysis was carried out with the results of Tax4Fun2, in which the relative abundance of specific functions or genes was determined. Figure 4a shows the main identified pathways involved in methanogenesis and other routes related to methane production: the methylotrophic methanogenesis pathway (1–4), the hydrogenotrophic methanogenesis pathway (5–11), the acetoclastic methanogenesis pathway (12–16), gene functions common to all methanogenic pathways (17–23), and the Wood–Ljungdahl pathway (24–32) related to the SAO process [45]. Figure 4b shows the predicted relative abundance of the functions identified in Fig. 4a for the methanogenic reactor in the four process configurations. It was found that the functions related to methane production from methanol and methylamine were only present in M2, probably related to its higher diversity of archaea.
a Identified pathways involved in methanogenesis and SAO. Functional genes were identified in the KEGG database. Modified from KEGG and Buhlmann et al. [46] . b Relative abundances of predicted metabolic functions related to methanogenesis and SAO in the methanogenic reactors
Functional Profile M1 vs. M2
The functional profile between the single- and the two-stage configurations (M1 vs. M2) was similar in all methanogenic pathways. This result indicates that single- and two-stage methane productions combine the hydrogenotrophic and acetoclastic pathways under mesophilic conditions in both configurations. The functions of the Wood–Ljungdahl pathway were also found in M1 and M2. SAOB uses this pathway in reverse (oxidative direction), promoting acetate decomposition into H2 and CO2, supporting the metabolism of hydrogenotrophic methanogens, and competing for acetate with acetoclastic methanogens [45]. The abundance of genes related to this pathway in M1 and M2 confirms the presence of microorganisms carrying out SAO. As previously suggested, in M1 and M2, this function is probably associated with abundant bacteria genera such as Syner-01. It has been reported that the presence of ammonium, acetate, temperature, retention time, and trace elements influence SAO [45, 46]. The presence of functions of the Wood–Ljungdahl pathway may also be related to the homoacetogenic pathway, which forms acetate from H2 and CO2 in the reductive direction [47]. The presence of homoacetogens supports the metabolism of acetoclastic methanogens and competes with hydrogenotrophic methanogens. The abundance of Wood–Ljungdahl pathway functions suggests that some homoacetogens could be present; however, their function could not be related to the most abundant genera in the single- and two-stage configurations under mesophilic conditions. It should be noted that the functional analysis allowed to support the hypotheses about the methanogenic pathways that yielded the taxonomic information of M1 and M2, but with a higher level of detail.
Functional Profile M2 vs. M3
Figure 4b shows that the functional profiles substantially differ between mesophilic and thermophilic conditions (M1 and M2 vs. M3 and M4). The abundance of genes related to hydrogenotrophic methanogenesis was higher in M3 than in M2, especially the enzyme 4Fe-4S ferredoxin that assimilates H2 and CO2. This result suggests that this pathway is fundamental in producing methane in thermophilic conditions, which is confirmed by the predominance of Methanothermobacter in M3. Interestingly, the enzyme acetyl-CoA synthetase (14) was highly abundant under thermophilic conditions, suggesting that this is the primary mechanism of acetate assimilation, which could be related to the presence of acetoclastic methanogenesis in M3. However, it has been reported that certain enzymes of acetoclastic methanogens are shared by bacteria performing SAO [46]. For this reason, a better explanation for the high predicted abundance of acetyl-CoA synthetase (14) and acetyl-CoA decarbonylase (15,16) is that they are part of the metabolic machinery of SAOB. Moreover, the abundance of formate dehydrogenase (30–32) indicates the presence of this other pathway by forming H2 and CO2 from formate.
Another interesting result is the low abundance of other enzymes related to the oxidative Wood–Ljungdahl pathway in thermophilic conditions (26–29). Due to its relative abundance in the taxonomic profile, the most likely candidate to perform SAO in M3 is Syntrophaceticus. Only one characterized species represents this genus, Syntrophaceticus schinkii, in which acetate oxidation proceeds via the Wood–Ljungdahl pathway [48]. Nevertheless, it has been suggested that SAOB can use other metabolic strategies than the Wood–Ljungdahl pathway [49], which may be the case of the Syntrophaceticus species identified in M3. This hypothesis, the possible alternate pathway followed by SAOB, arises from the functional analysis, which would not be possible with taxonomic information alone. However, validating this hypothesis and all those generated by functional inference requires the further use of other molecular tools, such as metatranscriptomics and metabolomics. Consequently, further studies are needed to understand the metabolic mechanisms and the ecological role of SAOB. Regardless of the mechanism, SAO may replace acetoclastic methanogenesis during the two-stage thermophilic digestion of winery effluents. Previous studies reported that this phenomenon has occurred in other thermophilic methanogenic systems in acetate-rich environments [45, 50]. It was recently suggested that a better comprehension of the factors affecting SAO could be used to design strategies to improve the anaerobic digestion processes [47].
Functional Profile M3 vs. M4
In order to finish the analysis of the functional profiles, Fig. 4b shows that the methanogenic reactor’s predicted metabolic functions were not significantly affected when HOC were applied (high-OLR, short-HRT). This result indicates that despite a loss of diversity and microorganisms genera between M3 and M4, the HOC did not affect the abundance of mechanisms for methane production. However, it is essential to mention that the loss of members of the microbial community could have affected other metabolic functions that were not in the scope of this analysis or even the rate of metabolic functions, which cannot be inferred with taxonomic and functional profiles. Further studies are needed to fully understand the effect of operating parameters on the stability of the process and the metabolism of its communities.
Functional Redundancy in the Methanogenic Reactors
The last stage of the analysis evaluated the FR in configurations 1 to 4 for the methanogenic reactors (Fig. S7). A total of 6881 functions are shared among the four configurations. The overall FR was higher in the two-stage configuration versus single-stage (M2 > M1); it was higher in mesophilic versus thermophilic conditions (M2 > M3), and it was lower after applying HOC (M3 > M4). The FR of the target metabolic functions related to methane production in the methanogenic reactors was compared (Fig.S8). The FR of these genes was 3 times higher (log ratio of 1.6) in the two-stage configuration compared to the single-stage configuration. Mesophilic conditions provided 2.5 times higher FR (log ratio of 1.3) than thermophilic conditions. Meanwhile, increased OLR and reduced HRT decreased the FR up to 1.5 times (log ratio of 0.54) on average. As mentioned above, FR is directly related to the degree of stability and resilience to disturbances in the process. These results indicate that the two-stage configuration provides higher functional stability and resilience than the single-stage configuration; the microbial community in mesophilic conditions has greater functional resilience than those in thermophilic conditions and applying HOC reduces the FR and stability of the process.
Potential Applications
Functional analysis, in general, and the information provided by this study have potential applications in anaerobic processes. Functional profiles offer insights into the key metabolic pathways and functional genes involved in methane production, enabling the identification of optimal process configurations and operating conditions. For example, this study identified that a two-stage configuration and mesophilic conditions were the most stable for treating winery effluents due to their higher FR. The analysis of functional profiles identified interactions and synergies among different microbial species present in the reactors, such as those observed between SAOB and hydrogenotrophic methanogens under thermophilic conditions. Understanding these microbial interactions is crucial for comprehending complex microbial communities and their responses to environmental changes, providing valuable insights for modeling microbial communities in anaerobic systems. These applications contribute to a deeper understanding and improved management of anaerobic processes.
Conclusions
This study showed that the higher stability and resilience of the two-stage configuration were caused by higher microbial diversity and FR compared to the single-stage configuration. Mesophilic conditions provided higher stability and functional resilience than thermophilic conditions, and applying HOC decreased the process stability. Metabolic specialization and a reduction in FR occurred in the acidogenic reactors, while SAO may play a vital role in the methanogenic reactors under thermophilic conditions. This analysis expands the knowledge of microbial diversity and its relation to microbial functions and process operating conditions, aiming to improve anaerobic process engineering.
Data Availability
The raw 16S rRNA gene sequences analyzed during the current study were deposited into the NCBI database under the Bioproject accession number PRJNA950123, with their corresponding metadata (http://www.ncbi.nlm.nih.gov/bioproject/950123).
References
Gudiukaite R, Nadda AK, Gricajeva A, Shanmugam S, Nguyen DD, Lam SS (2021) Bioprocesses for the recovery of bioenergy and value-added products from wastewater: a review. J Environ Manage 300:113831. https://doi.org/10.1016/j.jenvman.2021.113831
de e Silva ADS, Morais NWS, Coelho MMH, Pereira EL, dos Santos AB (2020) Potentialities of biotechnological recovery of methane hydrogen and carboxylic acids from agro-industrial wastewaters. Bioresour Technol Rep 10:100406. https://doi.org/10.1016/j.biteb.2020.100406
Vital-Jacome M, Cazares-Granillo M, Carrillo-Reyes J, Buitron G (2020) Characterization and anaerobic digestion of highly concentrated Mexican wine by-products and effluents. Water Sci Technol 81:190–198. https://doi.org/10.2166/wst.2020.102
Buitrón G, Martínez-Valdez FJ, Ojeda F (2019) Biogas production from a highly organic loaded winery effluent through a two-stage process. Bioenergy Res 12:714–721. https://doi.org/10.1007/s12155-019-09984-7
Carrillo-Reyes J, Albarrán-Contreras BA, Buitrón G (2019) Influence of added nutrients and substrate concentration in biohydrogen production from winery wastewaters coupled to methane production. Appl Biochem Biotechnol 187:140–151. https://doi.org/10.1007/s12010-018-2812-5
Villegas-Rodríguez S, Buitrón G (2021) Performance of native open cultures (winery effluents ruminal fluid anaerobic sludge and digestate) for medium-chain carboxylic acid production using ethanol and acetate. J Water Process Eng 40:101784. https://doi.org/10.1016/j.jwpe.2020.101784
Vital-Jacome MA, Buitrón G (2021) Thermophilic anaerobic digestion of winery effluents in a two-stage process and the effect of the feeding frequency on methane production. Chemosphere 272:129865. https://doi.org/10.1016/j.chemosphere.2021.129865
Li Y, Chen Y, Wu J (2019) Enhancement of methane production in anaerobic digestion process: a review. Appl Energy 240:120–137. https://doi.org/10.1016/j.apenergy.2019.01.243
Castellano-Hinojosa A, Armato C, Pozo C, González-Martínez A, González-López J (2018) New concepts in anaerobic digestion processes: recent advances and biological aspects. Appl Microbiol Biotechnol 102:5065–5076. https://doi.org/10.1007/s00253-018-9039-9
Pasalari H, Gholami M, Rezaee A, Esrafili A, Farzadkia M (2021) Perspectives on microbial community in anaerobic digestion with emphasis on environmental parameters: a systematic review. Chemosphere 270:128618. https://doi.org/10.1016/j.chemosphere.2020.128618
Cabezas A, de Araujo JC, Callejas C, Galès A, Hamelin J, Marone A, Sousa DZ, Trably E, Etchebehere C (2015) How to use molecular biology tools for the study of the anaerobic digestion process? Rev Environ Sci Biotechnol 14:555–593. https://doi.org/10.1007/s11157-015-9380-8
Djemiel C, Maron PA, Terrat S, Dequiedt S, Cottin A, Ranjard L (2022) Inferring microbiota functions from taxonomic genes: a review. GigaScience 11:1–30. https://doi.org/10.1093/gigascience/giab090
Li Y, Chen Z, Peng Y, Huang W, Liu J, Mironov V, Zhang S (2022) Deeper insights into the effects of substrate to inoculum ratio selection on the relationship of kinetic parameters microbial communities and key metabolic pathways during the anaerobic digestion of food waste. Water Res 217:118440. https://doi.org/10.1016/j.watres.2022.118440
Gao J, Liu G, Li H, Xu L, Du L, Yang B (2016) Predictive functional profiling using marker gene sequences and community diversity analyses of microbes in full-scale anaerobic sludge digesters. Bioprocess Biosyst Eng 39:1115–1127. https://doi.org/10.1007/s00449-016-1588-7
Greslehner GP (2020) Microbiome structure and function: a new framework for interpreting data. BioEssays 42:7. https://doi.org/10.1002/bies.201900255
Louca S, Jacques SMS, Pires APF, Leal JS, Srivastava DS, Parfrey LW, Farjalla VF, Doebeli M (2016) High taxonomic variability despite stable functional structure across microbial communities. Nat Ecol Evol 1:1–12. https://doi.org/10.1038/s41559-016-0015
Zheng Z, Cai Y, Zhang Y, Zhao Y, Gao Y, Cui Z, Hu Y, Wang X (2021) The effects of C/N (10–25) on the relationship of substrates metabolites and microorganisms in “inhibited steady-state” of anaerobic digestion. Water Res 188:116466. https://doi.org/10.1016/j.watres.2020.116466
Comeau AM, Li WKW, Tremblay JÉ, Carmack EC, Lovejoy C (2011) Arctic ocean microbial community structure before and after the 2007 record sea ice minimum. PLoS One 6:e27492. https://doi.org/10.1371/journal.pone.0027492
Parada AE, Needham DM, Fuhrman JA (2016) Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol 18:1403–1414. https://doi.org/10.1111/1462-2920.13023
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA (2016) Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13(7):581–583. https://doi.org/10.1038/nmeth.3869
Dueholm MKD, Nierychlo M, Andersen KS, Rudkjøbing V, Knutsson S, Arriaga S, Bakke R, Boon N, Bux F, Christensson M, Chua ASM, Curtis TP, Cytryn E, Erijman L, Etchebehere C, Fatta-Kassinos D, Frigon D, Garcia-Chaves MC, Gu AZ, Horn H, Jenkins D, Kreuzinger N, Kumari S, Lanham A, Law Y, Leiknes T, Morgenroth E, Muszyński A, Petrovski S, Pijuan M, Pillai SB, Reis MAM, Rong Q, Rossetti S, Seviour R, Tooker N, Vainio P, van Loosdrecht M, Vikraman R, Wanner J, Weissbrodt D, Wen X, Zhang T, Nielsen PH, Albertsen M, Nielsen PH (2022) MiDAS 4: a global catalogue of full-length 16S rRNA gene sequences and taxonomy for studies of bacterial communities in wastewater treatment plants. Nat Commun 13:1–15. https://doi.org/10.1038/s41467-022-29438-7
Wemheuer F, Taylor JA, Daniel R, Johnston E, Meinicke P, Thomas T, Wemheuer B (2020) Tax4Fun2: prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene sequences. Environ Microbiomes 15:1–12. https://doi.org/10.1186/s40793-020-00358-7
Buitrón G, Muñoz-Páez KM, Quijano G, Carrillo-Reyes J, Albarrán-Contreras BA (2020) Biohydrogen production from winery effluents: control of the homoacetogenesis through the headspace gas recirculation. J Chem Technol Biotechnol 95:544–552. https://doi.org/10.1002/jctb.6263
Nunes Ferraz Júnior AD, Pages C, Latrille E, Bernet N, Zaiat M, Trably E (2020) Biogas sequestration from the headspace of a fermentative system enhances hydrogen production rate and yield. Int J Hydrogen Energy 45:11011–11023. https://doi.org/10.1016/j.ijhydene.2020.02.064
Feng K, Li H, Zheng C (2018) Shifting product spectrum by pH adjustment during long-term continuous anaerobic fermentation of food waste. Bioresour Technol 270:180–188. https://doi.org/10.1016/j.biortech.2018.09.035
Zhang L, Ban Q, Li J, Wan C (2019) Functional bacterial and archaeal dynamics dictated by pH stress during sugar refinery wastewater in a UASB. Bioresour Technol 288:121464. https://doi.org/10.1016/j.biortech.2019.121464
García-Depraect O, Valdez-Vázquez I, Rene ER, Gómez-Romero J, López-López A, León-Becerril E (2019) Lactate- and acetate-based biohydrogen production through dark co-fermentation of tequila vinasse and nixtamalization wastewater: metabolic and microbial community dynamics. Bioresour Technol 282:236–244. https://doi.org/10.1016/j.biortech.2019.02.100
Bartowsky EJ, Henschke PA (2008) Acetic acid bacteria spoilage of bottled red wine—a review. Int J Food Microbiol 125:60–70. https://doi.org/10.1016/j.ijfoodmicro.2007.10.016
Chojnacka A, Błaszczyk MK, Szczesny P, Nowak K, Sumińska M, Tomczyk-Zak K, Zielenkiewicz U, Sikora A (2011) Comparative analysis of hydrogen-producing bacterial biofilms and granular sludge formed in continuous cultures of fermentative bacteria. Bioresour Technol 102:10057–10064. https://doi.org/10.1016/j.biortech.2011.08.063
Lagoa-Costa B, Kennes C, Veiga MC (2020) Cheese whey fermentation into volatile fatty acids in an anaerobic sequencing batch reactor. Bioresour Technol 308:123226. https://doi.org/10.1016/j.biortech.2020.123226
Moestedt J, Müller B, Nagavara Nagaraj Y, Schnürer A (2020) Acetate and lactate production during two-stage anaerobic digestion of food waste driven by Lactobacillus and Aeriscardovia. Front Energy Res 8:105. https://doi.org/10.3389/fenrg.2020.00105
Zhang L, Loh KC, Dai Y, Tong YW (2020) Acidogenic fermentation of food waste for production of volatile fatty acids: bacterial community analysis and semi-continuous operation. Waste Manage 109:75–84. https://doi.org/10.1016/j.wasman.2020.04.052
Yin Q, Wu G, Lens PNL (2022) Characterization of the core microbial community governing acidogenic processes for the production of valuable bioproducts. npj Clean Water 5(1):39. https://doi.org/10.1038/s41545-022-00180-3
Lim JW, Park T, Tong YW, Yu Z (2020) The microbiome driving anaerobic digestion and microbial analysis. Adv Bioener 5:1–61. https://doi.org/10.1016/bs.aibe.2020.04.001
Carvalho G, Pedras I, Karst SM, Oliveira CSS, Duque AF, Nielsen PH, Reis MAM (2018) Functional redundancy ensures performance robustness in 3-stage PHA-producing mixed cultures under variable feed operation. New Biotechnol 40:207–217. https://doi.org/10.1016/j.nbt.2017.08.007
Weimer PJ, Kohn RA (2016) Impacts of ruminal microorganisms on the production of fuels: how can we intercede from the outside? Appl Microbiol Biotechnol 100:3389–3398. https://doi.org/10.1007/s00253-016-7358-2
Harirchi S, Wainaina S, Sar T, Nojoumi SA, Parchami M, Parchami M, Varjani S, Khanal SK, Wong J, Awasthi MK, Taherzadeh MJ (2022) Microbiological insights into anaerobic digestion for biogas hydrogen or volatile fatty acids (VFAs): a review. Bioengineered 13(3):6521–6557. https://doi.org/10.1080/21655979.2022.2035986
Wang JJ, Xu LZJ, Huang BC, Li J, Jin RC (2021) Multiple electron acceptor-mediated sulfur autotrophic denitrification: nitrogen source competition long-term performance and microbial community evolution. Bioresour Technol 329:124918. https://doi.org/10.1016/j.biortech.2021.124918
Jo Y, Kim J, Hwang K, Lee C (2018) A comparative study of single- and two-phase anaerobic digestion of food waste under uncontrolled pH conditions. Waste Manage 78:509–520. https://doi.org/10.1016/j.wasman.2018.06.017
Maspolim Y, Zhou Y, Guo C, Xiao K, Ng WJ (2015) Determination of the archaeal and bacterial communities in two-phase and single-stage anaerobic systems by 454 pyrosequencing. J Environ Sci 36:121–129. https://doi.org/10.1016/j.jes.2015.02.017
Merlino G, Rizzi A, Schievano A, Tenca A, Scaglia B, Oberti R, Adani F, Daffonchio D (2013) Microbial community structure and dynamics in two-stage vs single-stage thermophilic anaerobic digestion of mixed swine slurry and market bio-waste. Water Res 47:1983–1995. https://doi.org/10.1016/j.watres.2013.01.007
Ao T, Xie Z, Zhou P, Liu X, Wan L, Li D (2021) Comparison of microbial community structures between mesophilic and thermophilic anaerobic digestion of vegetable waste. Bioprocess Biosyst Eng 44:1201–1214. https://doi.org/10.1007/s00449-021-02519-5
Wu ZL, Lin Z, Sun ZY, Gou M, Xia ZY, Tang YQ (2020) A comparative study of mesophilic and thermophilic anaerobic digestion of municipal sludge with high-solids content: reactor performance and microbial community. Bioresour Technol 302:122851. https://doi.org/10.1016/j.biortech.2020.122851
Peces M, Astals S, Jensen PD, Clarke WP (2021) Transition of microbial communities and degradation pathways in anaerobic digestion at decreasing retention time. New Biotechnol 60:52–61. https://doi.org/10.1016/j.nbt.2020.07.005
Westerholm M, Moestedt J, Schnürer A (2016) Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Appl Energy 179:124–135. https://doi.org/10.1016/j.apenergy.2016.06.061
Buhlmann CH, Mickan BS, Jenkins SN, Tait S, Kahandawala TKA, Bahri PA (2019) Ammonia stress on a resilient mesophilic anaerobic inoculum: methane production microbial community and putative metabolic pathways. Bioresour Technol 275:70–77. https://doi.org/10.1016/j.biortech.2018.12.012
Pan X, Zhao L, Li C, Angelidaki I, Lv N, Ning J, Cai G, Zhu G (2021) Deep insights into the network of acetate metabolism in anaerobic digestion: focusing on syntrophic acetate oxidation and homoacetogenesis. Water Res 190:116774. https://doi.org/10.1016/j.watres.2020.116774
Manzoor S, Bongcam-Rudloff E, Schnürer A, Müller B (2016) Genome-guided analysis and whole transcriptome profiling of the mesophilic syntrophic acetate oxidising bacterium Syntrophaceticus schinkii. PLoS One 11:e0166520. https://doi.org/10.1371/journal.pone.0166520
Mosbæk F, Kjeldal H, Mulat DG, Albertsen M, Ward AJ, Feilberg A, Nielsen JL (2016) Identification of syntrophic acetate-oxidizing bacteria in anaerobic digesters by combined protein-based stable isotope probing and metagenomics. ISME J 10(10):2405–2418. https://doi.org/10.1038/ismej.2016.39
Dyksma S, Jansen L, Gallert C (2020) Syntrophic acetate oxidation replaces acetoclastic methanogenesis during thermophilic digestion of biowaste. Microbiome 8:1–14. https://doi.org/10.1186/s40168-020-00862-5
Acknowledgements
The technical support of Gloria Moreno, Jaime Pérez, and Ángel A. Hernández is acknowledged.
Funding
This work was supported by DGAPA-UNAM (PAPIIT project TA101822) and Instituto de Ingeniería, UNAM (GII project no. 3406) and Consejo de Ciencia y Tecnología del Estado de Querétaro (project CACTI/90/2022).
Author information
Authors and Affiliations
Contributions
Miguel Vital-Jácome: conceptualization, methodology, formal analysis, and writing—reviewing and editing. Julián Carrillo-Reyes: formal analysis, reviewing, and editing. Germán Buitrón: reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vital-Jácome, M., Carrillo-Reyes, J. & Buitrón, G. Metabolic Functional Profiles of Microbial Communities in Methane Production Systems Treating Winery Wastewater. Bioenerg. Res. 17, 669–680 (2024). https://doi.org/10.1007/s12155-023-10633-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10633-3