Abstract
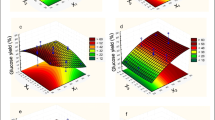
Pretreatment of biomass is one of the most challenging steps in the process of second-generation (2G) ethanol and biochemical production. Dilute acid pretreatment is a widely adapted and convenient method to recover pentose (C5) as well as hexose (C6) sugars due to its featured solubilization of hemicellulose and cellulose before and after enzymatic saccharification, respectively. In the present study, dilute sulfuric acid (H2SO4) pretreatment of sugarcane bagasse (SCB) was statistically optimized using the face-centered composite design (FCCD) of response surface methodology (RSM) in terms of acid concentration (0.1–3% v/v), solid loading (5–20% w/v) and residence time (15–60 min) at constant temperature of 121 °C followed by enzymatic hydrolysis using commercial cellulase (Novozymes Cellic CTec2) for enhanced combined sugar yield (CSY) comprising of C5 and C6 sugars in pretreated as well as saccharified hydrolysates. Optimized process parameters found in the study were 2.18% (v/v) acid; 14.35% (w/v) solid loading; and 29.49 min residence time. CSY under optimized conditions was found to be 521.42 ± 7.2 g/kg raw SCB with 72.06 ± 1.0% sugars recovered out of the maximum theoretical sugars present in the raw biomass. Total reducing sugar yields in pretreated and saccharified hydrolysates were found to be 215.28 ± 2.4 and 306.14 ± 5.3 g/kg raw SCB, respectively. Morphological and structural changes in optimized pretreated and saccharified biomass further validated the efficiency of optimized pretreatment applied in the present study. The maximum ethanol concentration, volumetric productivity and yield from released sugars were calculated as 10.82 ± 2.2 g/L, 0.45 ± 0.9 g/L/h and 0.42 g/g-glucose consumed or 71.45 ± 2.5 g/kg raw SCB, respectively. Ethanol yield obtained from the fermentation of dilute H2SO4-pretreated SCB was corresponding to 82.4% of the theoretical ethanol yield.





Similar content being viewed by others
Data availability
All data generated during this study are included in this published article.
References
Chandel AK, Albarelli JQ, Santos DT, Chundawat SP, Puri M, Meireles MAA (2019) Comparative analysis of key technologies for cellulosic ethanol production from Brazilian sugarcane bagasse at a commercial scale. Biofuels Bioprod Biorefining 13:994–1014. https://doi.org/10.1002/bbb.1990
Aditiya HB, Mahlia TMI, Chong WT, Nur H, Sebayang AH (2016) Second generation bioethanol production: a critical review. Renew Sust Energ Rev 66:631–653. https://doi.org/10.1016/j.rser.2016.07.015
Hans M, Garg S, Pellegrini VO, Filgueiras JG, de Azevedo ER, Guimaraes FE, Chandel AK, Polikarpov I, Chadha BS, Kumar S (2021) Liquid ammonia pretreatment optimization for improved release of fermentable sugars from sugarcane bagasse. J Clean Prod 281:123922. https://doi.org/10.1016/j.jclepro.2020.123922
Khaleghian H, Karimi K, Behzad T (2015) Ethanol production from rice straw by sodium carbonate pretreatment and Mucor hiemalis fermentation. Ind Crops Prod 76:1079–1085. https://doi.org/10.1016/j.indcrop.2015.08.008
Akhtar N, Goyal D, Goyal A (2016) Characterization of microwave-alkali-acid pre-treated rice straw for optimization of ethanol production via simultaneous saccharification and fermentation (SSF). Energy Convers Manag 141:133–144. https://doi.org/10.1016/j.enconman.2016.06.081
Miyamoto T, Mihashi A, Yamamura M, Tobimatsu Y, Suzuki S, Takada R, Kobayashi Y, Umezawa T (2018) Comparative analysis of lignin chemical structures of sugarcane bagasse pretreated by alkaline, hydrothermal, and dilute sulfuric acid methods. Ind Crops Prod 121:124–131. https://doi.org/10.1016/j.indcrop.2018.04.077
Soares IB, Mendes KCS, Benachour M, Abreu CAM (2017) Evaluation of the effects of operational parameters in the pretreatment of sugarcane bagasse with diluted sulfuric acid using analysis of variance. Chem Eng Comm 204:1369–1390. https://doi.org/10.1080/00986445.2017.1365061
Ávila-Lara AI, Camberos-Flores JN, Mendoza-Pérez JA, Messina-Fernández SR, Saldaña-Duran CE, Jimenez-Ruiz EI, Sánchez-Herrera LM, Pérez-Pimienta JA (2015) Optimization of alkaline and dilute acid pretreatment of agave bagasse by response surface. FrontBioengBiotechnol 3:1–10. https://doi.org/10.3389/fbioe.2015.00146
Benjamin YH, Cheng H, G€orgens JF (2014) Optimization of dilute sulfuric acid pretreatment to maximize combined sugar yield from sugarcane bagasse for ethanol production. ApplBiochem Biotechnol 172:610–630. https://doi.org/10.1007/s12010-013-0545-z
Sritrakul N, Nitisinprasert S, Keawsompong S (2017) Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agr Natl Resour 51:512–519. https://doi.org/10.1016/j.anres.2017.12.006
Arora R, Behera S, Sharma NK, Kumar S (2015) A new search for thermotolerant yeasts, its characterization and optimization using response surface methodology for ethanol production. Front Microbiol Microbiotechnol Ecotoxicol Bioremed 6:1–16. https://doi.org/10.3389/fmicb.2015.00889
Arora R, Behera S, Sharma NK, Kumar S (2017) Augmentation of ethanol production through statistically designed growth and fermentation medium using novel thermotolerant yeast isolates. Renew Energy 109:406–421. https://doi.org/10.1016/j.renene.2017.03.059
Sluiter A, Hames B, Hyman D, Payne C, Ruiz R, Scarlata C, Sluiter J, Templeton D, Wolfe J (2008) Determination of total solids in biomass and total dissolved solids in liquid process samples. National Renewable Energy Laboratory, U.S. Department of Energy, NREL/TP-510–42621a.
Sluiter A, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of Ash in Biomass. National Renewable Energy Laboratory, U.S. Department of Energy, NREL/TP-510–42622b.
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Int J Dairy Sci 74:3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Adney B, Baker J (1996). Measurement of Cellulase Activities. Chemical Analysis and Testing Task Laboratory Analytical Procedure, LAP-006.
Borgia GC, Brown RJS, Fantazzini P (1998) Uniform-Penalty inversion of multiexponential decay data. JMagn Reson 132:65–77. https://doi.org/10.1006/jmre.1998.1387
Bernardes A, Pellegrini VOA, Curtolo F, Camilo CM, Mello BL, Johns MA, Scott JL, Guimaraes FEC, Polikarpov I (2019) Carbohydrate binding modules enhance cellulose enzymatic hydrolysis by increasing access of cellulases to the substrate. Carbohyd Polym 211:57–68. https://doi.org/10.1016/j.carbpol.2019.01.108
Espirito Santo ME, Rezende CA, Bernardinelli OD Jr, NP, Curvelo AAS, de Azevedo ER, Guimarães FEG, Polikarpov I, (2018) Structural and compositional changes in sugarcane bagasse subjected to hydrothermal and organosolv pretreatments and their impacts on enzymatic hydrolysis. Ind Crops Prod 113:64–67. https://doi.org/10.1016/j.indcrop.2018.01.014
Tan YH, Abdullah MO, Nolasco-Hipolito C, Syuhada N, Zauzi A (2017) Application of RSM and Taguchi methods for optimizing the transesterification of waste cooking oil catalyzed by solid ostrich and chicken-eggshell derived CaO. Renew Energy 114:437–447
Ohale PE, Uzoh CF, Onukwuli OD (2017) Optimal factor evaluation for the dissolution of alumina from Azaraegbelu clay in acid solution using RSM and ANN comparative analysis. S Afr J Chem 24:43–54
Silveira MHL, Chandel AK, Vanelli BA, Sacilotto KS, Cardoso EB (2018) Production of hemicellulosic sugars from sugarcane bagasse via steam explosion employing industrially feasible conditions: Pilot scale study. Bioresour Technol Rep 3:138–146. https://doi.org/10.1016/j.biteb.2018.07.011
Hans M, Kumar S (2019) Biohythane production in two-stage anaerobic digestion system. Int J Hydrogen Energ 44:17363–17380
Roy S, Vishnuvardhan M, DasD (2014) Improvement of hydrogen production by newly isolated Thermoanaerobacterium thermosaccharolyticum IIT BT-ST1. Int JHydrogen Energ 39:7541–7552
Rajan K, Carrier DJ (2014) Effect of dilute acid pretreatment conditions and washing on the production of inhibitors and on recovery of sugars during wheat straw enzymatic hydrolysis. Biomass Bioenergy 62:222–227. https://doi.org/10.1016/j.biombioe.2014.01.013
Rojas-Rejón OA, Sánchez A (2014) The impact of particle size and initial solid loading on thermochemical pretreatment of wheat straw for improving sugar recovery. Bioproc Biosyst Eng 37:1427–1436
Canilha L, Chandel AK, dos Santos MTS, Fernandes Antunes FA, da Costa Freitas WL, das Grac M, FelipeA, da Silva SS (2012) Bioconversion of sugarcane biomass into ethanol: an overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J BiomedBiotechnol 1-15https://doi.org/10.1155/2012/989572
Gámez S, González-Cabriales JJ, Ramírez JA, Garrote G, Vázquez M (2006) Study of the hydrolysis of sugar cane bagasse using phosphoric acid. J Food Eng 74:8–88. https://doi.org/10.1016/j.jfoodeng.2005.02.005
Kumar S, Dheeran P, Singh SP, Mishra IM, Adhikari DK (2015) Kinetic studies of two-stage sulphuric acid hydrolysis of sugarcane bagasse. Renew Energy 83:850–858. https://doi.org/10.1016/j.renene.2015.05.033
Behera S, Arora R, Nandhagopal N, Kumar S (2014) Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renewe Sust Energ Rev 36:91–106
Deng L-H, Tang Y, Liu Y (2014) Detoxification of corncob acid hydrolysate with SAA pretreatment and xylitol production by immobilized Candida tropicalis. Sci World J.1–11.
Zhang H, Wu S (2014) Dilute ammonia pretreatment of sugarcane bagasse followed by enzymatic hydrolysis to sugars. Cellulose 21:1341–1349. https://doi.org/10.1007/s10570-014-0233-3
Chen Y, Wu Y, Zhu B, Zhang G, Wei N (2018) Co-fermentation of cellobiose and xylose by mixed culture of recombinant Sacchromyces cerevisae and kinetic modeling. PLoS ONE 13:19–25. https://doi.org/10.1371/journal.pone.0199104
Batalha LA, Han Q, Jameel H, Chang H-M, Colodette JL, Borges Gomes FJ (2015) Production of fermentable sugars from sugarcane bagasse by enzymatic hydrolysis after autohydrolysis and mechanical refining. Bioresour Technol 180:97–105. https://doi.org/10.1016/j.biortech.2014.12.060
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour Bioproc 4:1–19. https://doi.org/10.1186/s40643-017-0137-9
GuilhermeAA DPVF (2015) Evaluation of composition, Characterization and enzymatic hydrolysis of pretreated sugarcane bagasse. BrazJ Cheml Eng 32:23–33
Brienzo M, Fikizolo S, Benjamin Y, Tyhoda L, Görgens J (2017) Influence of pretreatment severity on structural changes, lignin content and enzymatic hydrolysis of sugarcane bagasse samples. Renew Energy 104:271–280
Sun XF, Xu F, Sun RC, FowlerP BMS (2005) Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohyd Res 340:97–106
Brienzo M, Tyhoda L, BenjaminY GörgensJ (2015) Relationship between physicochemical properties and enzymatic hydrolysis of sugarcane bagasse varieties for bioethanol production. New Biotechnol 32:253–262
Gabhane J, William SPMP, Vaidya AN, Mahapatra K, Chakrabart T (2011) Influence of heating source on the efficacy of lignocellulosic pretreatment: A cellulosic ethanol perspective. Biomass Bioenergy 35:96–102. https://doi.org/10.1016/j.biombioe.2010.08.026
Coletta VC, Rezende CA, da Conceição FR, Polikarpov I, Guimarães FEG (2013) Mapping the lignin distribution in pretreated sugarcane bagasse by confocal and fluorescence lifetime imaging microscopy. Biotechnol Biofuels 6:1–10
de Menezes FF, da Silva Fernandes RH, de Moraes Rocha GJ, Filhoc RM (2016) Physicochemical characterization of residue from the enzymatic hydrolysis of sugarcane bagasse in a cellulosic ethanol process at pilot scale. IndCrops Prod 94:463–470. https://doi.org/10.1016/j.indcrop.2016.09.014
Bernardinelli OD, Lima MA, Rezende CA, Polikarpov I, de Azevedo RE (2015) Quantitative 13C multi CP solid-state NMR as a tool for evaluation of cellulose crystallinity index measured directly inside sugarcane biomass. Biotechnol Biofuels 8:1–11. https://doi.org/10.1186/s13068-015-0292-1
Capitani D, TullioV Di, Proietti N (2012) Nuclear magnetic resonance to characterize and monitor cultural heritage. Prog Nucl Mag Res Spec 64:29–69
Borgia GC, Brown RJS, Fantazzini P (1998) Uniform-Penalty inversion of multiexponential decay data. J Mag Res 132:65–77
Sharma NK, Behera S, Arora R, Kumar S, Sani RK (2018) Xylose transport in yeast for lignocellulosic ethanol production: current status. J biosci bioeng 125(3):259–267
Navarrete C, Nielsen J, Siewers V (2014) Enhanced ethanol production and reduced glycerol formation in fps1Δ mutants of Saccharomyces cerevisiae engineered for improved redox balancing. AMB Express 4:86
Nissen TL, Hamann CW, Kielland-Brandt MC, Nielsen J, Villadsen J (2000) Anaerobic and aerobic batch cultivations of Saccharomyces cerevisiae mutants impaired in glycerol synthesis. Yeast 16(5):463–474
Iram M, Asghar U, Irfan M, Syed Q (2018) Production of bioethanol from sugarcane bagasse using yeast strains: a kinetic study. Energy 40(15):1–9
Boonchuay P, Techapun C, Leksawasdi N, Seesuriyachan P, Hanmoungjai P, Watanabe M, Takenaka S, Chaiyaso T (2018) An integrated process for xylooligosaccharide and bioethanol production from corncob. Bioresour Technol 256:399–407
Acknowledgements
Authors (Meenu Hans and Sachin Kumar) are very thankful to the Department of Biotechnology, Ministry of Science and Technology, India, for providing funds to carry out the research work via grant no. DBT/IC-2/Indo-Brazil/2016-19/05. One of the authors (Meenu Hans) is very thankful to Sardar Swaran Singh National Institute of Bio-Energy, Kapurthala for providing Senior Research Fellowship, and Guru Nanak Dev University, Amritsar for providing Ph.D. registration (Reg. No. 2007.KJ/A.519). We also acknowledge Novozymes, Denmark, for providing Cellic Ctec 2. Biophysical study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) via grants 405191/2015-4, 140667/2015-6, 158752/2015-5, 303988/2016-9 and 440977/2016-9 and by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) via grant#2015/13684-0. A.K. Chandel is thankful to the CAPES for visiting researcher and professor program. The SEM experiments were carried out with the assistance of Mr. Manoel Ricardo Roncon, São Carlos Institute of Physics, University of São Paulo.
Funding
Department of Biotechnology, Ministry of Science and Technology, India (DBT/IC-2/Indo-Brazil/2016–19/05); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (405191/2015–4, 140667/2015–6, 158752/2015–5, 303988/2016–9 and 440977/2016–9); and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2015/13684–0).
Author information
Authors and Affiliations
Contributions
MH conceived, planned, executed experiments and wrote original draft; VOAP, JGF, ERA and FECG analyzed biophysical parameters; AKC edited; IP reviewed and supervised; BSC reviewed; and SK conceived, planned, reviewed, edited and supervised.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors agree to publish the article in BERE.
Competing interests
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hans, M., Pellegrini, V.O.A., Filgueiras, J.G. et al. Optimization of Dilute Acid Pretreatment for Enhanced Release of Fermentable Sugars from Sugarcane Bagasse and Validation by Biophysical Characterization. Bioenerg. Res. 16, 416–434 (2023). https://doi.org/10.1007/s12155-022-10474-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10474-6