Abstract
Bermudagrass (Cynodon spp.) is a common perennial summer forage crop in the southeastern USA that could also be used for cellulosic ethanol. This study was conducted on two sites near Midville, GA to assess biomass yields, nutrient utilization, and cellulosic ethanol production from bermudagrass over 2 years (2010 and 2011). The crop was harvested either three times (June, midsummer, and fall), twice (midsummer and fall), or only once in the fall. The first harvest tended to yield the same amount of biomass regardless of season. In 2010, the three-cut system yielded the most biomass overall, while there was little difference in total yields between the three systems in 2011. The concentrations of N, P, and K in harvested biomass changed with age of the crop and harvest timing. Total N and K removal was greatest in the three-cut system, particularly in 2011. Biomass fiber properties were slightly affected by harvest timing, but these data did not show correlations with ethanol yield. Ethanol production was measured by subjecting biomass to dilute acid pretreatment and simultaneous saccharification and fermentation (SSF) at bench scale in the laboratory. Some variation in ethanol production was observed among treatments in 2011. Though harvest timing had minor effects on conversion of the biomass to ethanol, biomass yield is still the major factor that determines per hectare production of cellulosic ethanol from bermudagrass. Under the conditions in this study, a single harvest appears to be the most economical system for producing cellulosic ethanol from this crop. To maximize yield, two or three cuts should be possible, but much more N would be removed in the three-cut system, with little additional yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bermudagrass (Cynodon spp.) is an important forage crop in the southeast USA that could also be used for production of cellulosic ethanol [3]. A recent study by Anderson et al. [4] compared conversion of bermudagrass with that of napiergrass (Pennisetum purpureum Schum) and giant reed (Arundo donax L.). Bermudagrass was found to produce more ethanol per unit of dry matter (DM) than the other two grasses. Although the yield potential of bermudagrass is not nearly as high as these other grasses, growers are already familiar with managing bermudagrass for forage production, and several million hectares of bermudagrass hayfields and pastures are already in place. In addition, bermudagrass is easily dried and baled in the field, allowing for relatively easy transport and storage. Furthermore, bermudagrass can be harvested several times during the summer, whereas most other high-yielding energy grasses, such as switchgrass (Panicum virgatum L.) and giant miscanthus (Miscanthus × giganteus Greef et Deuter), would likely be harvested in the fall or winter after senescence to maximize yield and nutrient efficiency [9, 14, 15]. Thus, bermudagrass has the potential to fill a gap in the overall biomass harvesting season to allow for a more steady supply of biomass to the cellulosic ethanol refineries.
Generally, forage is harvested in such a way as to balance digestibility and protein content with biomass yield. Bermudagrass harvesting usually takes place at 3- to 5-week intervals, but it is not always possible to harvest the crop at the proper time, resulting in forage of reduced quality. Such low-quality forage could still be suitable for conversion to biofuel [3]. Additionally, older, more mature plant biomass may actually be better suited to biofuel conversion than younger forage, as the nutrient concentration should be lower in the older material. A recent study by Muir et al. [19] compared DM yields of bermudagrass harvested only once, as for bioenergy, versus every 21 days (14 total harvests), as would be done for forage, under a rainfed production system in Texas. The total DM yield was reduced by repeated harvests (8.28 Mg ha−1 year−1 for single cut, versus 5.74 Mg ha−1 year−1 for 14 total harvests), while the total N removal was much greater with multiple harvests (55.7 kg ha−1 year−1 for single cut versus 102.6 kg ha−1 year−1 for 14 harvests). Clearly, harvesting this frequently would not be suitable for biomass production, but perhaps two or three harvests could be appropriate. In Oklahoma, Haque et al. [9] reported that a two-harvest system (July and October) produced more bermudagrass biomass than a single harvest in October. The purpose of our study was to compare the biomass yield and nutrient utilization of bermudagrass under three different harvest systems: harvesting once, twice, or three times each season. Genetic differences related to cellulosic ethanol yield have been reported in bermudagrass [5], but less is known about the seasonal variation of this trait within a cultivar. Thus, the effects of harvest timing on cellulosic ethanol production were assessed directly using a bench-scale simultaneous saccharification and fermentation (SSF) procedure.
Materials and Methods
Experimental Design and Study Site
Sprigs of bermudagrass cultivar Tifton 85 [6] were planted at the Southeast Georgia Research and Education Center near Midville, GA, on 23 April 2009 on a Dothan loamy sand (fine loamy, siliceous, thermic Plinthic Kandiudults). The entire field was irrigated to establish the plants, after which the field was divided into two separate locations. Site 1 was maintained as rainfed only, while site 2 received irrigation. During the establishment year, the grass was only harvested three times late in the season, beginning in August (data not shown). In the spring of each year, granular ammonium nitrate fertilizer (112 kg ha−1 N) was surface-applied to all plots on both sites. Prior to planting, a soil test indicated approximately 23 mg kg−1 P and 46 mg kg−1 K at pH 6.3. Despite these relatively low readings, no P or K was applied during the study.
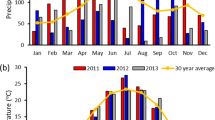
This study was conducted during the growing seasons (April–September) of 2010 and 2011. Monthly rainfall totals for site 1 and rainfall plus irrigation totals for site 2 are presented in Fig. 1. The total rainfall for site 1 from April through September was 53.9 cm in 2010 and 30.6 cm in 2011. Irrigation in site 2 began in April, around the time the bermudagrass began to show substantial growth. Irrigation was applied at a rate of approximately 1.9 cm (0.75 in.) per week during the 2010 growing season unless sufficient rainfall was received. In 2011, the rate was increased to approximately 2.5 cm (1 in.) per week during the warmer months of July, August, and September. Adding rainfall and irrigation, site 2 received a total of 87.6 and 82.5 cm of total rainfall equivalent in 2010 and 2011, respectively [21]. Each site had four replications arranged in a randomized complete block design. In 2010 and 2011, one plot from each replication was harvested three times (June, midsummer, and fall), another plot twice (midsummer and fall), and one plot was harvested only once (fall). The three-cut treatment was chosen to simulate hay production, while the two- and one-cut systems were chosen to simulate low-input biomass production. Due to weather and time constraints, the exact harvest dates varied slightly each year and are given in Table 1. Each plot consisted of two rows 1.5 m wide by 9 m long. The biomass was harvested into cloth bags with a self-propelled forage harvester (Carter Mfg. Co., Brookston, IN), and the fresh yields were recorded using a hanging scale. A sample from each plot was weighed fresh, dried to a stable weight in an oven at 60 °C, and weighed again to determine dry matter (DM) concentration. The dried samples were then ground in a Wiley mill to pass a 2-mm screen for further analyses.
Monthly precipitation totals (site 1) and precipitation plus irrigation (site 2) recorded near Midville, GA during the growing seasons of years 2010 and 2011 [21]
Analysis of Biomass Nutrients and Fiber Properties
Concentrations of nutrients in dried, ground biomass samples were determined at the University of Georgia Agricultural and Environmental Services Laboratories (AESL). Nitrogen concentration was determined by dry combustion, and P and K concentrations by inductively coupled plasma (ICP) spectrometry. Total nutrient removals were calculated by multiplying nutrient concentrations by DM yields.
Ground biomass samples were also subjected to standard forage fiber analyses. Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined using F57 Fiber Filter Bags (ANKOM Technology, Macedon, NY) in an A2000 Fiber Analyzer (ANKOM Technology) according to the manufacturer’s protocols. Acid detergent lignin (ADL) was determined using a Daisy II incubator (ANKOM Technology). Hemicellulose concentration was approximated by subtracting ADF from NDF, and cellulose concentration was approximated by subtracting ADL from ADF [20].
Biomass Pretreatment and Simultaneous Saccharification and Fermentation
Biomass from three replications each year was used to estimate conversion of bermudagrass biomass to ethanol using a benchtop dilute acid pretreatment and SSF procedure as described by Doran-Peterson et al. [8] with minor modifications. Samples of biomass were dried overnight in an oven at 70 °C to determine exact moisture concentrations to correct for moisture absorbed during storage (usually around 50 g kg−1). Two-gram DM was then placed in a 125-ml Erlenmeyer flask with 10 ml dilute sulfuric acid (1.75 % w/v H2SO4). Based on moisture content of the biomass, enough water was added to make a total volume of 14.7 ml. Each flask was capped with a rubber stopper, which was vented with a hypodermic needle. This was then autoclaved for 1 h at 121 °C (liquid cycle). Each pretreatment/SSF was carried out in duplicate. After pretreatment, samples were cooled to room temperature, and then the pH was adjusted to 5.0 using approximately 1.7 ml 10 % w/v Ca(OH)2 and 0.6 ml 1 M citric acid. The pH was checked using pHydrion paper (Micro Essential Laboratory, Inc., Brooklyn, NY), and any further pH adjustments were made using drops of 5 M KOH or HCl. Enzymes were mixed with 10× YP broth (2 ml flask−1) and then filter-sterilized with a disposable filter system (Corning, Inc., Corning, NY). Each SSF reaction contained 10 FPU Celluclast 1.5 L cellulase (Novozymes, Franklinton, NC) and 120 CBU Novo 188 cellobiase (Novozymes). Activity of the cellulase was determined using the procedure described by Adney and Baker [1]. Activity of the cellobiase was obtained from the manufacturer. Xylose-fermenting yeast (Saccharomyces cerevisiae) strain YRH400 [10] was added to an OD600 of 0.5 and a final fermentation volume of 20 ml. The SSFs were placed in an incubator/shaker at 30 °C with constant agitation at 150 rpm. Samples (approx. 1 ml) were taken every 24 h to quantify ethanol and reducing sugars. Samples were centrifuged to remove insoluble material, and then the supernatant was filtered through a CoStar Spin-X microcentrifuge tube 0.22 μm nylon filter (Corning, Inc.) to remove all remaining yeast cells and stop fermentation. Samples were then immediately frozen (−20 °C) until analysis.
Quantification of Reducing Sugars and Ethanol
Reducing sugars were quantified using the dinitrosalicylic acid (DNS) method [17], modified for PCR plates as described by King et al. [12]. Five microliters of filtered sample was reacted with 145 μl DNS reagent at 95 °C for 5 min in a PTC-100 thermocycler (MJ Research, Waltham, MA). Twenty microliters of each reaction was then diluted into 160 μl deionized water in clear-bottom microplates, and absorbance at 540 nm was measured in a SpectraFluor Plus (Tecan Group, Ltd., Männedorf, Switzerland) fitted with a 540-nm bandpass filter (Omega Optical, Inc., Brattleboro, VT). A standard curve was constructed using known concentrations of glucose.
Ethanol was quantified by mixing equal volumes of filtered sample with a 2 % (v/v) aqueous solution of isopropanol (internal standard). One to two microliters of this mixture was injected into a GC-17A gas chromatograph (Shimadzu Corp., Kyoto, Japan) fitted with a DB-WAX column (30 m × 0.53 mm ID, 1 μm film thickness; Agilent Technologies, Inc., Santa Clara, CA), with a constant oven temperature of 50 °C, injector and detector temperatures of 230 °C, and a column flow rate of 2.8 ml He min−1. The peak area ratio of ethanol/internal standard was used for ethanol quantification based on a standard curve from known concentrations of ethanol.
Statistical Procedures
Data were analyzed in SAS v. 9.2 (SAS Institute, Cary, NC) using the GLIMMIX procedure. Year, location, and year × location were included in all models as fixed factors, each contributing one degree of freedom. Replications (within location) were treated as a random factor with plots designated as the subjects within the RANDOM statement. Overdispersed data, such as biomass yields, were transformed using the DIST = LOGNORMAL option in the MODEL statement to obtain a better-fitting model. Tukey’s HSD test was used to determine significant differences between LS means at α ≤ 0.05. The CORR procedure was used to test for correlations between dependent variables.
Results
Biomass Yield
More rainfall was received in 2010 than in 2011 (Fig. 1), which likely explains the year effect and year by location interaction effect that were observed for biomass yield (Tables 2 and 3). In 2010, biomass yields did not differ between the two sites, but there were some differences between the different harvest regimes (Table 4). All of the first harvests, regardless of the season, yielded essentially the same quantity of DM, averaging 11.29 Mg ha−1. Second and third harvests were very similar to each other, but were much lower than the first cuttings, with a mean of 3.54 Mg ha−1. The three-cut system produced the most DM overall (16.02 Mg ha−1 at site 1 and 20.04 Mg ha−1 at site 2), though at site 1 the total DM yield of the three-cut system was not different than the two-cut system (13.30 Mg ha−1). In the drier year (2011), yields at site 2 were greater than at site 1 (p < 0.001), but the harvest treatment effect was the same. Yields of the first cutting were greater than subsequent cuttings, and as in the previous year, the yield of the first cutting was not affected by season. The mean DM yield for the first cutting was 8.60 Mg ha−1 at site 1 and 13.11 Mg ha−1 at site 2. A second cutting, whether in midsummer or fall, yielded the same quantity of biomass (2.67 Mg ha−1 at site 1 and 5.76 Mg ha−1 at site 2), and the third cutting yield was less than that of the second cuttings. In 2011, the third cutting yielded only 0.79 Mg ha−1 at site 1 and 3.56 Mg ha−1 at site 2 (Table 4). Surprisingly, the total DM yields of the three harvest systems did not differ in 2011 at either location.
Nutrient Uptake
Harvest timing greatly affected biomass N concentrations, but the effect differed between years 2010 and 2011 (Table 2). Biomass N concentrations did not differ between locations in 2010, but they tended to be higher in site 1 in 2011 (p < 0.001). In 2010, the greatest N concentration was found in the June harvest, with a mean of 15.3 g kg−1. Delaying the first harvest into midsummer or fall resulted in decreased N concentration. The lowest N concentration was found in the single fall harvest (8.7 g kg−1; Table 5). In 2010, regrowth (second and third harvests) in the three-harvest system had lower N concentration than the first harvest in June. However, in the two-harvest system, the regrowth (second harvest) had similar N concentration as the first harvest (Table 5). In 2011, the second and third harvests in the three-cut system had the highest concentrations of N (17.1 g kg−1 for site 1 and 13.7 g kg−1 for site 2). Also in 2011, delaying the first harvest did not affect N concentration at site 1, but at site 2, delaying the first harvest from June to fall resulted in a decrease of 3.2 g kg−1 of biomass N concentration (Table 5).
Delaying the first harvest generally did not affect biomass P concentration, except at site 1 in 2010, where the first midsummer harvest (two-cut system) had 0.61 g kg−1 lower P concentration than the June harvest. Phosphorus concentration tended to increase in the regrowth (second and third cuttings) in both years, though not always significantly (Table 5). Delaying the first harvest also resulted in decreased biomass K concentration in both 2010 and 2011. For example, in 2010, the mean K concentration was 18.2 g kg−1 in June, but dropped to a mean of 14.4 g kg−1 for a midsummer first cutting. A further delay into the fall did not further decrease biomass K concentration in 2010. However, in 2011, a delay of the first harvest from June until midsummer did not decrease biomass K concentration, but a delay from June to fall resulted in a decrease of 7.0 g kg−1 at site 1 and a decrease of 4.6 g kg−1 at site 2. (Biomass K concentration was greater at site 1 in 2011; p = 0.001). Regrowth in the three-harvest system had lower K concentration than the June harvest in 2010 at site 1, but in all other cases, regrowth had similar K concentration as the first harvest in both the three-cut and two-cut systems (Table 5).
In 2010, total N removal was not different between locations, but it was greater at site 2 in 2011. In 2010, the three-harvest system removed an average of 252 kg ha−1 N (Table 6), which is 177 and 232 % of what was removed by the two- or one-harvest systems, respectively. In 2011, total N removal was not different between harvest systems at site 1. At site 2, the three-cut system removed 243 kg ha−1 N, more than the one-cut system at 135 kg ha−1. However, the two-cut system (186 kg ha−1 N) was not different from either the three-cut or one-cut system (Table 6).
In both years, P removal tended to be greater at site 2, but harvest regimen only affected P removal in 2010. At both sites in 2010, the three-cut system removed more P than the two-cut or one-cut systems (Table 6). Potassium removal showed a similar pattern as N removal. In 2010, the three-harvest system removed 305 kg ha−1 K, compared to 189 and 149 kg ha−1 for the two-cut and one-cut systems, respectively, with no difference between locations. In 2011, K removal was not affected by harvest regimen at site 1, but at site 2, it was lowest in the one-cut system, at 180 kg ha−1, compared to 314 and 279 kg ha−1 for the three-cut and two-cut systems, respectively (Table 6).
Biomass Fiber Properties and Ethanol Yield
Harvest timing had some effects on biomass fiber properties in both 2010 and 2011. Neutral detergent fiber concentration was more variable among harvests in 2010, with the highest NDF concentration in the midsummer harvest of the two-cut system. In 2011, NDF was not affected by harvest, except that the fall single harvest had higher NDF than any other treatment (Table 7). In 2010, ADF increased by 32 g kg−1 as the first harvest was delayed from June to midsummer, but then did not increase further. In 2011, ADF did not increase as the first harvest was delayed from June to midsummer, but did increase 23 g kg−1 as the first cutting was delayed from midsummer to fall (Table 7). Regrowth in the two-cut system (second harvest fall) also had lower ADF than the first harvest in this system in 2010. Surprisingly, ADL did not vary by harvest in either year. Hemicellulose concentration also did not vary by harvest in 2010 and only showed minor differences among harvests in 2011. Cellulose concentration increased as the first harvest was delayed from June to midsummer in 2010 and also increased in 2011 as the first harvest was delayed from midsummer to fall. This pattern is similar to that observed for ADF, of which cellulose is the major component.
In 2010, ethanol yield ranged from 128 to 141 g kg−1 DM; however, this was not a sufficient variation to detect any differences. This differed from the results in 2011, where ethanol yield ranged between 128 and 149 g kg−1 DM, and differences were detected between harvest treatments. Biomass from the fall harvest of the three-cut treatment produced the least ethanol per unit of DM (128 g kg−1), while biomass from the fall harvest of the two-cut treatment and the first harvests from the two- and three-cut systems produced the most ethanol per unit of DM (Table 7). Ethanol yields showed no correlations between biomass fiber properties (data not shown).
Discussion
Total N removal generally exceeded the amount applied (112 kg ha−1 year−1), except for the single-harvest system in 2010 and the single or two-harvest systems at site 1 in 2011, all of which removed approximately the amount of N that was applied. Alderman et al. [2] reported that application of N after cutting enhanced regrowth, but only at rates up to 90 kg ha−1 cutting−1. However, application of N after cutting also increased the N concentration of the biomass. This is ideal for forage, but probably not for bioenergy purposes, and would increase production costs. Nonetheless, the N removal rates observed here suggest that a slightly higher application rate will be needed to sustain production for two- or three-harvest systems. Based on removal rates (Table 6), K fertilizer would need to be applied in the range of 150–300 kg ha−1 year−1 depending on the intended harvest system. Potassium application is important in stand persistence of bermudagrass [11, 22]. Despite the low initial soil test values for P and K, it is possible that residual nutrients deep in the soil profile could sustain the crop for several more years before increased fertilization is needed. Bermudagrass roots have been observed as deep as 244 cm [7, 22], and even some sandy soils can contain a substantial supply of native K. In a study on similar soils (Clarendon and Alapaha loamy sands), Knoll et al. [13] reported that unfertilized napiergrass, a high-yielding perennial, removed 336 kg ha−1 N and 1,012 kg ha−1 K in the first 2 years of biomass production before yields started to decline.
A previous study of diverse accessions of bermudagrass by Anderson et al. [5] showed negative correlations between cellulosic ethanol production and NDF (r = −0.53), pentose sugars (r = −0.60), and Klason lignin (r = −0.45). However, ethanol yield in this study was not correlated with any biomass fiber properties. One explanation could be the relatively narrow range of values observed for NDF (738–775 g kg−1), ADF (355–404 g kg−1), and ADL (43.3–53.5 g kg−1) in this study compared to a previous work. In this study, only one cultivar was evaluated, and even the youngest forage harvested in this study was still quite mature at 40 days old (third harvest, fall in 2010; Table 1). In 2011, ethanol yield showed a moderate correlation (R = 0.432, p = 0.009) with release of reducing sugars by the dilute acid pretreatment, but this correlation was not observed in the 2010 data (not shown). The enzymes continue to release sugars during the fermentation process so that initial sugar release by pretreatment is not necessarily a good predictor of final ethanol yield. In all cases, some unfermented sugars remained after 72 h of SSF. The formation of inhibitory compounds, such as furfural and hydroxymethyl furfural (HMF), during dilute acid pretreatment has been documented [16, 18] and could be one explanation for incomplete fermentation. These remaining sugars may also be unfermentable sugars such as arabinose or oligosaccharides derived from the hemicellulose portion of the biomass.
The ethanol yields reported here are similar to those reported by Anderson et al. [4] for Tifton 85 bermudagrass harvested in November after about 11 weeks of regrowth and subjected to similar pretreatment and SSF conditions. Under the conditions described in this study, a single fall harvest of Tifton 85 bermudagrass would yield around 2,044 l ha−1 ethanol (219 US gal acre−1). Under irrigation or plentiful rainfall, a two- or three-harvest system would produce around 3,220 l ha−1 ethanol (345 US gal acre−1). These yields could obviously be increased by more efficient pretreatment, conversion, and fermentation conditions.
Conclusions
In this study, a two- or three-cut system maximized biomass yield of Tifton 85 bermudagrass. However, the three-cut system removed much more N and K than the two-cut system, making it less desirable economically. Under similar conditions, a single-harvest system would be the most efficient with respect to N usage. This single harvest could be done at any point throughout the summer, or delayed into fall, as relatively little additional growth was made after June in the single-harvest plots. This would allow for a more steady supply of biomass to ethanol refineries when biomass supplies might otherwise be low. Though changes in the quality of the biomass were observed over the course of the season, their overall effect on total ethanol yields appears to be relatively minor compared to overall biomass yields.
Abbreviations
- ADF:
-
Acid detergent fiber
- ADL:
-
Acid detergent lignin
- CBU:
-
Cellobiose unit
- DM:
-
Dry matter
- DNS:
-
3,5-Dinitrosalicylic acid
- FPU:
-
Filter paper unit
- NDF:
-
Neutral detergent fiber
- SSF:
-
Simultaneous saccharification and fermentation
References
Adney B, Baker J (1996) Measurement of cellulase activities, laboratory analytical procedure. U.S. Dept. of Energy, National Renewable Energy Laboratory, Golden
Alderman PD, Boote KJ, Sollenberger LE (2011) Regrowth dynamics of ‘Tifton 85’ bermudagrass as affected by nitrogen fertilization. Crop Sci 51:1716–1726
Anderson WF, Casler MD, Baldwin BS (2008) Improvement of perennial forage species as feedstock for bio-energy. In: Vermerris W (ed) Genetic improvement of bioenergy crops. Springer, New York, pp 309–346
Anderson WF, Dien BS, Brandon SK, Doran-Peterson J (2008) Assessment of bermudagrass and bunch grasses as feedstock for conversion to ethanol. Appl Biochem Biotechnol 145:13–21
Anderson WF, Dien BS, Jung HJG, Vogel KP, Weimer PJ (2010) Effects of forage quality and cell wall constituents of bermuda grass on biochemical conversion to ethanol. Bioenergy Res 3:225–237
Burton GW, Gates RN, Hill GM (1993) Registration of Tifton-85 bermudagrass. Crop Sci 33:644–645
Burton GW, DeVane EH, Carter RL (1954) Root penetration, distribution and activity in southern grasses measured by yields, drought symptoms and P32 uptake. Agron J 46:229–233
Doran-Peterson J, Jangid A, Brandon SK, DeCrescenzo-Henriksen E, Dien B, Ingram LO (2009) Simultaneous saccharification and fermentation and partial saccharification and co-fermentation of lignocellulosic biomass for ethanol production. In: Mielenz JA (ed) Biofuels: methods and protocols. Meth. Mol Biol 581:263–280
Haque M, Epplin FM, Taliaferro CM (2009) Nitrogen and harvest frequency effect on yield and cost for four perennial grasses. Agron J 101:1463–1469
Hector RE, Dien BS, Cotta MA, Qureshi N (2011) Engineering industrial Saccharomyces cerevisiae strains for xylose fermentation and comparison for switchgrass conversion. J Ind Microbiol Biotechnol 38:1193–1202
Keisling TC, Rouquette FM Jr, Matocha JE (1979) Potassium fertilization influences on Coastal bermudagrass rhizomes, roots, and stand. Agron J 71:892–894
King BC, Donnelly MK, Bergstrom GC, Walker LP, Gibson DM (2009) An optimized microplate assay system for quantitative evaluation of plant cell wall-degrading enzyme activity of fungal culture extracts. Biotechnol Bioeng 102:1033–1044
Knoll JE, Anderson WF, Strickland TC, Hubbard RK, Malik R (2012) Low-input production of biomass from perennial grasses in the coastal plain of Georgia, USA. Bioenergy Res 5:206–214
Lemus R, Parrish DJ, Wolf DD (2009) Nutrient uptake by ‘Alamo’ switchgrass used as an energy crop. Bioenergy Res 2:37–50
Lewandowski I, Heinz A (2003) Delayed harvest of miscanthus—influences on biomass quantity and quality and environmental impacts of energy production. Eur J Agron 19:43–63
Martin C, Alriksson B, Sjöde A, Nilvebrant NO, Jönsson LJ (2007) Dilute sulfuric acid pretreatment of agricultural and agro-industrial residues for ethanol production. Appl Biochem Biotechnol 136–140:339–352
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Muir JP, Lambert BD, Greenwood A, Lee A, Riojas A (2010) Comparing repeated forage bermudagrass harvest data to single, accumulated bioenergy feedstock harvests. Bioresour Technol 101:200–206
Theander O, Westerlund E (1993) Quantitative analysis of cell wall components. In: Jung HG, Buxton DR, Hatfield RD, Ralph J (eds) Forage cell wall structure and digestibility. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, pp 83–104
University of Georgia, Automated Environmental Monitoring Network. Available at: http://www.griffin.uga.edu/aemn/. Accessed 12 Feb 2013
Woodhouse WW Jr (1968) Long term fertility requirements of coastal bermudagrass, I. potassium. Agron J 60:508–512
Acknowledgments
The authors would like to thank Robert Pippin and Anthony Black (University of Georgia) for assistance in planting, maintaining, and harvesting the field plots. Freddie Cheek and Tony Howell (USDA-ARS) also assisted with harvesting. We would also like to acknowledge Melissa Tawzer (University of Georgia) for assistance with the fiber analyses, and we are grateful to Ron Hector and Bruce Dien (USDA-ARS) for providing the xylose-fermenting yeast (YRH400). Thanks to Dr. Dennis Hancock (University of Georgia) for helpful suggestions to improve this manuscript and to Dr. Zaid Abdo (USDA-ARS) for statistical assistance. This research was funded by U.S. Department of Energy award DE-FG36-08G088036.
Disclaimer
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Knoll, J.E., Johnson, J.M., Lee, R.D. et al. Harvest Management of ‘Tifton 85’ Bermudagrass for Cellulosic Ethanol Production. Bioenerg. Res. 7, 1112–1119 (2014). https://doi.org/10.1007/s12155-014-9449-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-014-9449-1