Abstract
Purpose
This study aimed to investigate the frequency of COVID-19 vaccine-induced reactive change and potential factors including blood type correlated with increased FDG uptake on positron emission tomography (PET)/computed tomography (CT).
Materials and methods
We evaluated 284 patients who underwent PET/CT between June and September 2021 and had a known history of COVID-19 vaccination. Information on the injection site, vaccine type, and adverse reactions was obtained. We visually assessed the presence or absence of accumulation in the axillary and supraclavicular lymph nodes and the deltoid muscles. We measured the maximum standardized uptake value (SUVmax) using semi-quantitative analysis.
Results
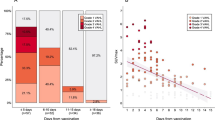
Our study included 158 males and 126 females aged 16–94. The median time between vaccination and PET/CT was 9 and 42 days for patients who had received their first and second doses, respectively. We observed axillary lymph node accumulation, supraclavicular lymph node accumulation, and deltoid muscle accumulation in 98 (SUVmax 1.07–25.1), nine (SUVmax 2.28–14.5), and 33 cases (SUVmax 0.93–7.42), respectively. In cases with axillary lymph node (P = 0.0057) or deltoid muscle (P = 0.047) accumulation, the shorter the time since vaccination, the higher the FDG accumulation. Patients with axillary lymph node accumulation were significantly younger (P < 0.0001) and had a significantly higher frequency of adverse reactions such as fever (P < 0.0001) and myalgia (P = 0.002). No significant relationship was observed between blood type and the frequency of FDG accumulation. Logistic regression analysis also showed that age, gender, days since vaccination, and adverse reactions such as fever and myalgia were important factors for axillary lymph node accumulation.
Conclusion
Our study found that FDG accumulation in the axillary lymph nodes and deltoid muscle was higher within a shorter time after vaccination, and axillary lymph node accumulation was higher in young patients, females, and those with adverse reactions of fever and myalgia. No significant relationship was observed between blood type and the frequency of FDG accumulation. Confirming the vaccination status, time since vaccination, and the presence of adverse reactions before PET may reduce false positives.


Similar content being viewed by others
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author upon request.
References
Gao P, Liu J, Liu M. Effect of COVID-19 vaccines on reducing the risk of long COVID in the real world: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(19):12422.
Samkowski J, Sklinda K, Walecki JM. Lymphadenopathy in the era of COVID-19 vaccination—an oncological dilemma in diagnostic imaging. Pol J Radiol. 2022;87:e304–10.
Okuyama C, Higashi T, Ishizu K, Saga T. FDG-PET findings associated with various medical procedures and treatments. Jpn J Radiol. 2022;41:1–18.
Culverwell AD, Scarsbrook AF, Chowdhury FU. False-positive uptake on 2-[18F]-fluoro-2-deoxy-D-glucose (FDG) positron-emission tomography/computed tomography (PET/CT) in oncological imaging. Clin Radiol. 2011;66(4):366–82.
Bshesh K, Khan W, Vattoth AL, Janjua E, Nauman A, Almasri M, et al. Lymphadenopathy post-COVID-19 vaccination with increased FDG uptake may be falsely attributed to oncological disorders: a systematic review. J Med Virol. 2022;94(5):1833–45.
Eifer M, Eshet Y. Imaging of COVID-19 vaccination at FDG PET/CT. Radiology. 2021;299(2):E248.
Becker AS, Perez-Johnston R, Chikarmane SA, Chen MM, El Homsi M, Feigin KN, et al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging. Radiology. 2021;300(2):E323–7.
Nawwar AA, Searle J, Hagan I, Lyburn ID. COVID-19 vaccination induced axillary nodal uptake on [18F]FDG PET/CT. Eur J Nucl Med Mol Imaging. 2021;48(8):2655–6.
Nawwar AA, Searle J, Singh R, Lyburn ID. Oxford-AstraZeneca COVID-19 vaccination induced lymphadenopathy on [18F]Choline PET/CT-not only an FDG finding. Eur J Nucl Med Mol Imaging. 2021;48(8):2657–8.
Avner M, Orevi M, Caplan N, Popovtzer A, Lotem M, Cohen JE. COVID-19 vaccine as a cause for unilateral lymphadenopathy detected by 18F-FDG PET/CT in a patient affected by melanoma. Eur J Nucl Med Mol Imaging. 2021;48(8):2659–60.
Ah-Thiane L, Ferrer L, Maucherat B, Fleury V, Le Thiec M, Rusu D, et al. Vaccine-related lymph nodes: the emerging pitfalls of 18F-fluorocholine and 68Ga-PSMA-11 PET/CT in the era of COVID-19 vaccination. Clin Nucl Med. 2022;47(7):575–82.
Skawran S, Schiesser H, Maurer A, Sartoretti T, Dittli M, Mader C, et al. Frequency and temporal evolution of COVID-19 vaccination rate among oncological patients undergoing 18F-FDG-PET. Vaccine. 2022;40(52):7640–5.
Eifer M, Pinian H, Klang E, Alhoubani Y, Kanana N, Tau N, et al. FDG PET/CT radiomics as a tool to differentiate between reactive axillary lymphadenopathy following COVID-19 vaccination and metastatic breast cancer axillary lymphadenopathy: a pilot study. Eur Radiol. 2022;32(9):5921–9.
Bhimaniya S, Jahromi A. Resolution of misleading COVID-19 vaccination-related nodal and splenic FDG uptake in the follow-up study. Clin Nucl Med. 2022;47(10):e658–9.
van Nijnatten TJA, Jochelson MS, Lobbes MBI. Axillary lymph node characteristics in breast cancer patients versus post-COVID-19 vaccination: overview of current evidence per imaging modality. Eur J Radiol. 2022;152: 110334.
Otomi Y, Irahara S, Inoue H, Shinya T, Otsuka H, Harada M. Increased. Mol Imaging Radionucl Ther. 2022;31(2):169–71.
Ayati N, Evans S, Zakavi SR, Gruenewald SM. Comparison between viral vector and mRNA based COVID-19 vaccination in prevalence and severity of regional immune reactions, and. Asia Ocean J Nucl Med Biol. 2023;11(1):4–12.
Andresciani F, Ricci M, Grasso RF, Zobel BB, Quattrocchi CC. COVID-19 vaccination simulating lymph node progression in a patient with prostate cancer. Radiol Case Rep. 2022;17(9):2996–9.
Rubin R. Investigating whether blood type is linked to COVID-19 risk. JAMA. 2020;324(13):1273.
Liu N, Zhang T, Ma L, Zhang H, Wang H, Wei W, et al. The impact of ABO blood group on COVID-19 infection risk and mortality: a systematic review and meta-analysis. Blood Rev. 2021;48: 100785.
Check D, Check JH, Kaplan N. The ABC’s (Autoimmunity, blood type, cytokines) in types and severity of reactions to COVID-19 vaccines. J Med Clin Res Rev. 2021;5(4):1–7.
Kaneta T, Ogawa M, Motomura N, Iizuka H, Arisawa T, Hino-Shishikura A, et al. Initial evaluation of the Celesteion large-bore PET/CT scanner in accordance with the NEMA NU2–2012 standard and the Japanese guideline for oncology FDG PET/CT data acquisition protocol version 2.0. EJNMMI Res. 2017;7(1):83.
Zhang M, Ahn RW, Hayes JC, Seiler SJ, Mootz AR, Porembka JH. Axillary lymphadenopathy in the COVID-19 era: what the radiologist needs to know. Radiographics. 2022;42(7):1897–911.
Shirone N, Shinkai T, Yamane T, Uto F, Yoshimura H, Tamai H, et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26(3):248–52.
Skawran S, Gennari AG, Dittli M, Treyer V, Muehlematter UJ, Maurer A, et al. [18F]FDG uptake of axillary lymph nodes after COVID-19 vaccination in oncological PET/CT: frequency, intensity, and potential clinical impact. Eur Radiol. 2022;32(1):508–16.
Li J, Wang X, Chen J, Cai Y, Deng A, Yang M. Association between ABO blood groups and risk of SARS-CoV-2 pneumonia. Br J Haematol. 2020;190(1):24–7.
Miotto M, Di Rienzo L, Gosti G, Milanetti E, Ruocco G. Does blood type affect the COVID-19 infection pattern? PLoS ONE. 2021;16(5): e0251535.
Alessa MY, Aledili FJ, Alnasser AA, Aldharman SS, Al Dehailan AM, Abuseer HO, et al. The side effects of COVID-19 vaccines and its association with ABO blood type among the general surgeons in Saudi Arabia. Cureus. 2022;14(3): e23628.
Allan JD, McMillan D, Levi ML. COVID-19 mRNA vaccination, ABO blood type and the severity of self-reported reactogenicity in a large healthcare system: a brief report of a cross-sectional study. Cureus. 2021;13(12): e20810.
Almalki OS, Khalifa AS, Alhemeidi OF, Ewis AA, Shady AM, Abdelwahab SF. Correlation between the severity of COVID-19 vaccine-related adverse events and the blood group of the vaccinees in Saudi Arabia: a web-based survey. Front Pharmacol. 2022;13:1006333.
Almalki OS, Santali EY, Alhothali AA, Ewis AA, Shady A, Fathelrahman AI, et al. The role of blood groups, vaccine type, and gender in predicting the severity of side effects among university students receiving COVID-19 vaccines. BMC Infect Dis. 2023;23(1):378.
Kubota K, Saginoya T, Ishiwata K, Nakasato T, Munechika H. [18F]FDG uptake in axillary lymph nodes and deltoid muscle after COVID-19 mRNA vaccination: a cohort study to determine incidence and contributing factors using a multivariate analysis. Ann Nucl Med. 2022;36(4):340–50.
El-Sayed MS, Wechie GN, Low CS, Adesanya O, Rao N, Leung VJ. The incidence and duration of COVID-19 vaccine-related reactive lymphadenopathy on. Clin Med (Lond). 2021;21(6):e633–8.
Maglione A, Rolla S, Mercanti SF, Cutrupi S, Clerico M. The adaptive immune system in multiple sclerosis: an estrogen-mediated point of view. Cells. 2019;8(10):1280.
Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–9.
Maeda K, Amano M, Uemura Y, Tsuchiya K, Matsushima T, Noda K, et al. Correlates of neutralizing/SARS-CoV-2-S1-binding antibody response with adverse effects and immune kinetics in BNT162b2-vaccinated individuals. medRxiv. 2021.
Cohen D, Krauthammer SH, Wolf I, Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed. Eur J Nucl Med Mol Imaging. 2021;48(6):1854–63.
Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Eifer M. Prevalence of increased FDG PET/CT axillary lymph node uptake beyond 6 weeks after mRNA COVID-19 vaccination. Radiology. 2021;300(3):E345–7.
Wolfson S, Kim E, Plaunova A, Bukhman R, Sarmiento RD, Samreen N, et al. Axillary adenopathy after COVID-19 vaccine: no reason to delay screening mammogram. Radiology. 2022;303(2):297–9.
Advani P, Chumsri S, Pai T, Li Z, Sharma A, Parent E. Temporal metabolic response to mRNA COVID-19 vaccinations in oncology patients. Ann Nucl Med. 2021;35(11):1264–9.
Mukai K, Tsunoda H, Imai R, Numata A, Kida K, Oba K, et al. The location of unilateral axillary lymphadenopathy after COVID-19 vaccination compared with that of metastasis from breast cancer without vaccination. Jpn J Radiol. 2023;41:1–8.
Cohen D, Hazut Krauthammer S, Wolf I, Even-Sapir E. A sigh of relief: vaccine-associated hypermetabolic lymphadenopathy following the third COVID-19 vaccine dose is short in duration and uncommonly interferes with the interpretation of [18F]FDG PET-CT studies performed in oncologic patients. Eur J Nucl Med Mol Imaging. 2022;49(4):1338–44.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
KT and OM conceived and designed the study. The material preparation, data collection, and analyses were performed by KT and OM. KT and OM wrote the first draft of this manuscript. All authors commented on the previous versions of the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Noriko Oyama-Manabe receives payments for lectures from Bayer Yakuhin, Ltd. and Canon Medical Systems. The other authors declare no financial conflicts of interest.
Ethical approval
Approval from our institutional ethics review board was obtained, and the requirement for informed consent was waived for this retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takahashi, K., Manabe, O., Shizukuishi, K. et al. Examination of iatrogenic FDG accumulation after COVID-19 vaccination. Ann Nucl Med (2024). https://doi.org/10.1007/s12149-024-01909-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12149-024-01909-5