Abstract
Objective
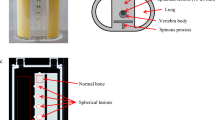
We previously developed a custom-design thoracic bone scintigraphy-specific phantom (“SIM2 bone phantom”) to assess image quality in bone single-photon emission computed tomography (SPECT). We aimed to develop an automatic assessment system for imaging technology in bone SPECT and demonstrate the validity of this system.
Methods
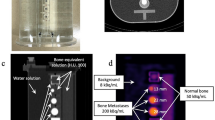
Four spherical lesions of 13-, 17-, 22-, and 28-mm diameters in the vertebrae of SIM2 bone phantom simulating the thorax were filled with radioactivity (target-to-background ratio: 4). Dynamic SPECT acquisitions were performed for 15 min; reconstructions were performed using ordered subset expectation maximization at 3–15-min timepoints. Consequently, 216 lesions (54 SPECT images) were obtained: 120 and 96 lesions were used for software development and validation, respectively. The developed software used statistical parametric mapping to rigidly register and automatically calculate quantitative indexes (contrast-to-noise ratio, % coefficient of variance, % detectability equivalence volume, recovery coefficient, target-to-normal bone ratio, and full width at half maximum). A detectability score (DS) was used to define the four observation types (4, excellent; 3, adequate; 2, average; 1, poor) to score hot spherical lesions. The gold standard for DSs was independently classified by three experienced board-certified nuclear medicine technologists using the four observation types; thereafter, a consensus regarding the gold standard for DSs was reached. Using 120 lesions for development, decision tree analysis was performed to determine DS based on the quantitative indexes. We verified the validation of the quantitative indexes and their threshold values for automatic classification using 96 lesions for validation.
Results
The trends in the automatically calculated quantitative indices were consistent. Decision tree analysis produced four terminal groups; two quantitative indexes (% detectability equivalence volume and contrast-to-noise ratio) were used to classify DS. The automatically classified DSs exhibited an almost perfect agreement with the gold standard. The percentage agreement and kappa coefficient were 91.7% and 0.93, respectively, in 96 lesions for validation.
Conclusions
The developed software automatically classified the detectability of hot lesions in the SIM2 bone phantom using the automatically calculated quantitative indexes, suggesting that this software could provide a means to automatically perform detectability analysis after data input that is excellent in reproducibility and accuracy.





Similar content being viewed by others
References
Schirrmeister H, Glatting G, Hetzel J, Nüssle K, Arslandemir C, Buck AK, et al. Prospective evaluation of the clinical value of planar bone scans, SPECT, and 18F-labeled NaF PET in Newly diagnosed lung cancer. J Nucl Med. 2001;42(12):1800–4.
Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-Fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47(2):287–97.
Abikhzer G, Gourevich K, Kagna O, Israel O, Frenkel A, Keidar Z. Whole-body bone SPECT in breast cancer patients: the future bone scan protocol? Nucl Med Commun. 2016;37(3):247–53.
Romer W, Nomayr A, Uder M, Bautz W, Torsten K. SPECT-guided CT for evaluating foci of increased bone metabolism classified as indeterminate on SPECT in cancer patients. J Nucl Med. 2006;47(7):1102–6.
Ichikawa H, Miwa K, Okuda K, Shibutani T, Kato T, Nagaki A, et al. Current state of bone scintigraphy protocols and practice in Japan. Asia Ocean J Nucl Med Biol. 2020;8(2):116–22.
Sadik M, Suurkula M, Hoglund P, Jarund A, Edenbrandt L. Quality of planar whole-body bone scan interpretations–a nationwide survey. Eur J Nucl Med Mol Imaging. 2008;35(8):1464–72.
Rose A. Vision. 1 ed: Springer US; 1973.
Carlier T, Eugène T, Bodet-Milin C, Garin E, Ansquer C, Rousseau C, et al. Assessment of acquisition protocols for routine imaging of Y-90 using PET/CT. EJNMMI Res. 2013;3(1):11.
Beijst C, de Keizer B, Lam M, Janssens GO, Tytgat GAM, de Jong H. A phantom study: Should (124) I-mIBG PET/CT replace (123) I-mIBG SPECT/CT? Med Phys. 2017;44(5):1624–31.
Alqahtani MS, Lees JE, Bugby SL, Samara-Ratna P, Ng AH, Perkins AC. Design and implementation of a prototype head and neck phantom for the performance evaluation of gamma imaging systems. EJNMMI Phys. 2017;4(1):19.
Adler S, Seidel J, Choyke P, Knopp MV, Binzel K, Zhang J, et al. Minimum lesion detectability as a measure of PET system performance. EJNMMI Phys. 2017;4(1):13.
Oen SK, Aasheim LB, Eikenes L, Karlberg AM. Image quality and detectability in Siemens Biograph PET/MRI and PET/CT systems-a phantom study. EJNMMI Phys. 2019;6(1):16.
Beijst C, Kist JW, Elschot M, Viergever MA, Hoekstra OS, de Keizer B, et al. Quantitative comparison of 124I PET/CT and 131I SPECT/CT detectability. J Nucl Med. 2016;57(1):103–8.
Ichikawa H, Kato T, Shimada H, Watanabe Y, Miwa K, Matsutomo N, et al. Detectability of thoracic bone scintigraphy evaluated using a novel custom-designed phantom. The Jpn J Nucl Med Technol. 2017;37(3):229–38.
Cachovan M, Vija AH, Hornegger J, Kuwert T. Quantification of (99m)Tc-DPD concentration in the lumbar spine with SPECT/CT. EJNMMI Res. 2013;3:45.
Kaneta T, Ogawa M, Daisaki H, Nawata S, Yoshida K, Inoue T. SUV measurement of normal vertebrae using SPECT/CT with Tc-99m methylene diphosphonate. Am J Nucl Med Mol Imaging. 2016;6(5):262–8.
Kuji I, Yamane T, Seto A, Yasumizu Y, Shirotake S, Oyama M. Skeletal standardized uptake values obtained by quantitative SPECT/CT as an osteoblastic biomarker for the discrimination of active bone metastasis in prostate cancer. Eur J Hybrid Imaging. 2017;1(1):2.
Dreuille OD, Strijckmans V, Ameida P, Loc’h C, Bendriem B. Bone equivalent liquid solution to assess accuracy of transmission measurements in SPECT and PET. IEEE Trans Nucl Sci. 1997;44(3):1186–90.
Zhang Z. Decision tree modeling using R. Ann Transl Med. 2016;4(15):275.
Dickerscheid D, Lavalaye J, Romijn L, Habraken J. Contrast-noise-ratio (CNR) analysis and optimisation of breast-specific gamma imaging (BSGI) acquisition protocols. EJNMMI Res. 2013;3(1):21.
Takahashi A, Himuro K, Baba S, Yamashita Y, Sasaki M. Comparison of TOF-PET and bremsstrahlung SPECT images of Yttrium-90: A Monte Carlo simulation study. Asia Ocean J Nucl Med Biol. 2018;6(1):24–31.
Dietze MMA, Kunnen B, van der Velden S, Steenbergen JHL, Koppert WJC, Viergever MA, et al. Performance of a dual-layer scanner for hybrid SPECT/CBCT. Phys Med Biol. 2019;64(10):105020.
Claus JJ, van Harskamp F, Breteler MMB, Krenning EP, van der Cammen TJM, Hofman A, et al. Assessment of cerebral perfusion with single-photon emission tomography in normal subjects and in patients with Alzheimer’s disease: effects of region of interest selection. Eur J Nucl Med. 1994;21(10):1044–51.
Liu HG, Mountz JM, Inampudi C, San Pedro EC, Deutsch G. A semiquantitative cortical circumferential normalization method for clinical evaluation of rCBF brain SPECT. Clin Nucl Med. 1997;22(9):596–604.
Palmedo H, Marx C, Ebert A, Kreft B, Ko Y, Turler A, et al. Whole-body SPECT/CT for bone scintigraphy: diagnostic value and effect on patient management in oncological patients. Eur J Nucl Med Mol Imaging. 2014;41(1):59–67.
Zacho HD, Manresa JAB, Aleksyniene R, Ejlersen JA, Fledelius J, Bertelsen H, et al. Three-minute SPECT/CT is sufficient for the assessment of bone metastasis as add-on to planar bone scintigraphy: prospective head-to-head comparison to 11-min SPECT/CT. EJNMMI Res. 2017;7(1):1.
Miyaji N, Miwa K, Tokiwa A, Ichikawa H, Terauchi T, Koizumi M, et al. Phantom and clinical evaluation of bone SPECT/CT image reconstruction with xSPECT algorithm. EJNMMI Res. 2020;10(1):71.
Acknowledgements
The authors would like to thank Yoshinao Misu for supporting the data analysis.
Funding
This study was supported by FUJIFILM Toyama Chemical, Co. Ltd, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hajime Ichikawa has a collaborative research work for developing software with FUJIFILM Toyama Chemical, Co. Ltd, Tokyo, Japan. Kazunori Kawakami, Kazuki Nagatake, and Tetsuo Hosoya are employees of FUJIFILM Toyama Chemical Co., Ltd. All other authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ichikawa, H., Kawakami, K., Onoguchi, M. et al. Automatic quantification package (Hone Graph) for phantom-based image quality assessment in bone SPECT: computerized automatic classification of detectability. Ann Nucl Med 35, 937–946 (2021). https://doi.org/10.1007/s12149-021-01631-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01631-6