Abstract
Objective
Immunotherapy for programmed cell death 1 (PD-1) and its ligand, PD-L1, has been considered an effective treatment for ovarian cancer. 18F-labeled fluoro-2-deoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is a widely used noninvasive imaging tool for diagnosing several cancers. In this study, we investigated the association between PD-L1 expression and the maximum standardized uptake value (SUVmax) using 18F-FDG PET/CT.
Methods
We retrospectively analyzed clinical data of patients with ovarian cancer who underwent 18F-FDG PET/CT. Patients were categorized into two groups according to PD-L1 expression results. The relationship between clinicopathological characteristics of patients with ovarian cancer and PD-L1 expression was examined.
Results
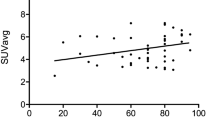
SUVmax was significantly higher in PD-L1-positive tumors than in PD-L1-negative tumors (16.1 ± 5.2 and 12.7 ± 7.0, respectively; p = 0.026). There were no significant differences in age, histologic type, and tumor grade between the PD-L1-negative and PD-L1-positive groups. The receiver operating characteristic curve analysis demonstrated that the highest accuracy (61.8%) for predicting PD-L1 expression was obtained with an SUVmax cutoff value of 10.5.
Conclusion
There was a significant correlation between 18F-FDG uptake and PD-L1 expression, suggesting a role of 18F-FDG PET/CT in selecting ovarian cancer candidates for anti-PD-L1 antibody therapy.



Similar content being viewed by others
References
Kaja S, Hilgenberg JD, Collins JL, et al. Detection of novel biomarkers for ovarian cancer with an optical nanotechnology detection system enabling label-free diagnostics. J Biomed Opt. 2012;17:081412–21.
Girolimetti G, Perrone AM, Santini D, et al. BRCA-associated ovarian cancer: from molecular genetics to risk management. Biomed Res Int. 2014;2014:787143.
du Bois A, Reuss A, Pujade-Lauraine E, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115:1234–44.
Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87.
D’Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112:95–102.
Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7.
Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62.
Thierauf J, Veit JA, Affolter A, et al. Identification and clinical relevance of PD-L1 expression in primary mucosal malignant melanoma of the head and neck. Melanoma Res. 2015;25:503–9.
Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5.
Cha YJ, Kim HR, Lee CY, et al. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung Cancer. 2016;97:73–80.
Tomioka N, Azuma M, Ikarashi M, et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC). Breast Cancer. 2018;25:34–42.
Jung HI, Jeong D, Ji S, et al. Overexpression of PD-L1 and PD-L2 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res Treat. 2017;49:246–54.
Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–66.
Kaira K, Higuchi T, Naruse I, et al. Metabolic activity by (18)F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45:56–66.
Jadvar H, Alavi A, Gambhir SS. 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med. 2009;50:1820–7.
Iagaru AH, Mittra ES, McDougall IR, et al. 18F-FDG PET/CT evaluation of patients with ovarian carcinoma. Nucl Med Commun. 2008;29:1046–51.
Prakash P, Cronin CG, Blake MA. Role of PET/CT in ovarian cancer. Am J Roentgenol. 2010;194:W464–70.
Takada K, Toyokawa G, Tagawa T, et al. Association between PD-L1 expression and metabolic activity on (18)F-FDG PET/CT in patients with small-sized lung cancer. Anticancer Res. 2017;37:7073–82.
Zhang M, Wang D, Sun Q, et al. Prognostic significance of PD-L1 expression and (18)F-FDG PET/CT in surgical pulmonary squamous cell carcinoma. Oncotarget. 2017;8:51630–40.
Sun WY, Lee YK, Koo JS. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J Transl Med. 2016;14:173.
Kim HR, Ha SJ, Hong MH, et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6:36956.
Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Awad MM, Hammerman PS. Durable responses with PD-1 inhibition in lung and kidney cancer and the ongoing search for predictive biomarkers. J Clin Oncol. 2015;33:1993–4.
Gordan JD, Lal P, Dondeti VR, et al. HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008;14:435–46.
Zhang H, Lu C, Fang M, et al. HIF-1alpha activates hypoxia-induced PFKFB4 expression in human bladder cancer cells. Biochem Biophys Res Commun. 2016;476:146–52.
Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90.
Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–41.
Funding
No source of funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, Y.J., Jo, K., Hwang, S.H. et al. Association between PD-L1 expression and 18F-FDG uptake in ovarian cancer. Ann Nucl Med 35, 415–420 (2021). https://doi.org/10.1007/s12149-020-01571-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-020-01571-7