Abstract
Purpose
To evaluate fluorine-18 fluorocholine (FCH) PET/CT for the detection of recurrent prostate cancer in relation to prostate-specific antigen (PSA) level.
Methods
FCH PET/CT was performed in 50 patients with rising PSA levels at follow-up of primary treatment of prostate cancer (radical prostatectomy in 28, radiation therapy in 13, and brachytherapy in 9). PET detection rates were determined at various PSA thresholds and examined by receiver operating characteristic analysis.
Results
Findings consistent with recurrent prostate cancer were noted on FCH PET/CT in 31/50 (62 %) patients, with positive findings in 17/18 (94 %), and 11/13 (85 %), 2/7 (29 %), and 1/12 (8 %) patients with PSA >4, >2–4, >0.5–2, and ≤0.5 ng/mL, respectively. These findings were indicative of local/regional recurrence in 23 cases and systemic recurrence in 8 cases, with only a single route of recurrence (i.e., either hematogenous, lymphatic, or intraprostatic) in 84 % of PET scans with positive findings. Abnormal tumor activity was detected in 88 % of patients with a PSA level of 1.1 ng/mL or higher, and in only 6 % of patients with a PSA level below this threshold value.
Conclusion
FCH PET/CT may serve to identify the route of tumor progression in patients with recurrent prostate cancer; however, the likelihood of tumor detection may be related to the PSA level at the time of imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A rising serum prostate-specific antigen (PSA) level is often the first indicator of recurrent disease in patients with prostate cancer treated with radiation therapy (RT) or radical prostatectomy (RP). At 5 years following such primary treatments, rising PSA levels can be detected in approximately 15–20 % of low-risk patients, and approximately 50 % of high-risk (i.e., Gleason score 8–10, PSA >20 ng/mL, stage T3) patients [1, 2]. Localizing recurrent tumors in these patients has proven to be a significant challenge when using conventional diagnostic imaging, particularly when the PSA level has not yet risen substantially [3–5]. While recurrence patterns in prostate cancer are known to vary by treatment [6, 7], awareness of these patterns is not sufficient to support the customization of salvage therapy for recurrent prostate cancer. Patient-individualized treatment approaches for recurrent prostate cancer could be enhanced if tumor spread along local, lymphatic, and hematogenous routes are correctly identified shortly after a rise in PSA is detected.
PET imaging using tracer analogs of choline has shown significant promise for detecting prostate cancer [8–13]. The rationale for using choline derivatives as oncologic PET tracers is based on the observation that choline transport and phosphorylation are upregulated in most cancers, including prostate cancer [14–17]. While there are some data on the performance of choline-based PET imaging for whole-body staging of prostate cancer, some uncertainty remains as to its diagnostic sensitivity in patients with low levels of PSA following RP, external beam RT, and interstitial brachytherapy. The purpose of this study is to evaluate the detection rate of FCH PET/CT in patients with rising PSA levels following primary treatment of prostate cancer, paying particular attention to the routes of tumor progression that can be identified as well as the potential relationship between PSA level and tumor detection.
Methods
Patient selection
Fifty sequential patients with a history of clinically localized prostate cancer who were noted to have an elevated PSA level >0.2 ng/mL on at least two serial measurements after completion of RP, RT, or interstitial brachytherapy were recruited to undergo FCH PET/CT imaging in this institutional review board-approved clinical research study. All patients gave written informed consent before their participation. Hormone-naïve patients as well as patients who received luteinizing hormone-releasing hormone (LHRH) agonist therapy for at least 3 months prior to the detection of an elevated PSA, including those treated with an LHRH agonist in conjunction with primary treatment, were eligible for this study. However, patients in whom LHRH agonist therapy was started recently, namely within 3 months, or following the detection of an elevated PSA level were excluded from the study.
Radiopharmaceutical synthesis and PET imaging
FCH synthesis was performed by fluorination of ditosylmethane with fluorine-18 followed by alkylation of the fluorotosylmethane intermediate with dimethylethanolamine using a chemical process control unit (CTI/Siemens CPCU, CTI/Siemens, Knoxville, TN) [18]. All synthesis products passed standard assays for radiochemical purity, radionuclidic identity, chemical purity, and pyrogenicity prior to injection. Radiochemical purity was >99 %.
PET/CT imaging
Patients refrained from eating or drinking for at least 3 h prior to PET/CT. Scans were performed using a Philips Gemini TF-64 PET/CT scanner (Philips Healthcare, Andover, MA). A CT transmission scan was first performed in the supine position from the pelvis to the skull. The 64-channel helical CT scanning parameters were: 120 kV, 50 mA/slice, rotation time 0.75 s, slice thickness/interval 5.0 mm. No intravenous contrast was used for CT. At approximately 12–15 min after intravenous injection of 2.6 MBq/kg (0.07 mCi/kg) of FCH, emission scans were acquired from mid-thigh to skull over 9–11 bed positions at 2 min per bed position. Image reconstruction employed a list-mode version of a maximum likelihood expectation maximization algorithm. CT data were used for attenuation correction.
Image interpretation and analysis
PET/CT images were visually assessed by two readers (SK, a nuclear medicine physician; MC, a radiologist). Both have extensive experience in the interpretation of whole-body FCH PET/CT. All PET/CT images were interpreted initially without access to the clinical data. The scans were classified as positive if a focal area of increased FCH uptake was visually identified in the bone, lymph node, visceral organ, or prostate gland (e.g., Fig. 1). The finding of increased uptake in inguinal, hilar or axillary nodes was an exception to this criterion, given that increased FCH uptake in benign reactive lymph nodes is known to occur [11]. The maximum standardized uptake value (SUVmax), defined as the highest ratio of measured activity to injected activity normalized to body weight, was recorded for each corresponding lesion by region of interest analysis.
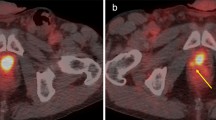
FCH PET/CT of recurrent prostate cancer after interstitial brachytherapy. a Axial PET image through the pelvis demonstrates focal increased activity in the prostatic region (arrow). b The corresponding CT image reveals the presence of brachytherapy implants in the prostate without other significant structural abnormality. c On PET/CT, images through the prostatic region localize to the area of increased FCH uptake to the left seminal vesicle and basal prostate (arrow). A subsequent biopsy of the left prostatic base confirms the presence of recurrent prostate cancer
Findings on FCH PET/CT were compared to clinical pathology and imaging data available at the time of imaging, as well as data obtained prospectively that included subsequent biopsy results, imaging studies and serial PSA measurements. In addition to using histology as the standard of reference, non-histologic criteria for recurrent metastatic prostate cancer were employed and based on radiographic findings of either (1) a marrow-based lesion showing abnormal signal intensity or enhancement on MRI, (2) evidence of bone destruction or invasion by tumor on CT scan, or (3) tumor enlargement on serial CT or MRI coinciding with a rising PSA. All patients also received a bone scintigraphic scan with Tc-99m-oxidronate (Technescan HDP, Mallinckrodt) within 2 months of enrollment. These scans were classified as unremarkable, non-specific, or consistent with metastatic disease based on their clinical interpretation.
Statistical analysis
Contingency tables were tested for significance using the Fisher exact test. Correlations were assessed by the Pearson product moment coefficient. Group differences were assessed by ANOVA. Receiver operating characteristic analysis was used to assess optimal cutoff points for PSA to predict positive findings on PET/CT. All tests were two tailed. Statistical analyses were performed using JMP 9 and SAS v 9.2 (SAS Institute, Inc., Cary, NC).
Results
Clinical data
Patient clinical data are summarized in Table 1. The median time interval between primary therapy and PSA recurrence was 48 months (range 3–219 months). The median PSA level at the time of initial PET/CT scanning was 3.2 ng/dL (mean 5.2, range 0.2–18.1). PSA levels did not differ significantly between RP, RT, and brachytherapy-treated patients (mean ± SD; 4.3 ± 1.0, 4.5 ± 1.4, and 5.6 ± 1.7, respectively, p = 0.68). PSA values did not differ significantly between LHRH agonist-treated and -untreated patients (7.1 and 4.2 ng/mL, respectively, p = 0.33).
FCH PET/CT findings
FCH PET/CT demonstrated positive findings in 31/50 (62 %) of patients. There was inter-observer agreement in the classification of a positive FCH PET/CT scan in all cases. Biopsy results were available to confirm the status of abnormal FCH PET/CT findings in 10/31 of the positive PET cases. Radiographic follow-up with serial bone scintigraphy, CT, and MRI for up to 6 months was successful in classifying all remaining FCH PET/CT results. PET/CT findings were consistent with local or regional recurrence in 23 cases and systemic recurrence in 8 cases. The abnormal findings on FCH PET/CT involved only a single organ system (i.e., the skeletal system, lymph nodes, or prostate) in 26/31 (84 %) of patients with positive scans. Positive findings occurred in 17/18 (94 %), 11/13 (85 %), 2/7 (29 %), and 1/12 (8 %) of patients with PSA >4, >2–4, >0.5–2, and ≤0.5 ng/mL, respectively (Fisher exact p = 2.79E−07). The proportion of positive FCH PET/CT findings in patients receiving LHRH agonist therapy was not significantly different than in untreated patients (10/15 (81 %) and 22/35 (63 %), respectively, p = 0.757).
The optimal PSA threshold for discriminating FCH PET/CT scans with positive findings was determined by receiver operating characteristic analysis to be 1.1 ng/mL. Based on this threshold, 88 % (30/34) of the patients with a PSA ≥1.1 ng/mL had positive PET/CT results (Fig. 2). The only FCH PET/CT that was positive below this threshold corresponded to a patient with PSA level of 0.3 ng/mL in whom a single retroperitoneal lymph node was detected on the basis of increased FCH uptake on PET/CT (Fig. 3). Thus, diagnostic sensitivity of FCH PET/CT on a per patient basis in this study was 88 % in patients with a PSA level of 1.1 ng/mL or higher.
Isolated retroperitoneal lymph node detected at a PSA of 0.3 ng/mL. a An 11-mm diameter retroperitoneal lymph node (arrowhead) with maximum SUV of 2.7 was the only abnormality found on FCH PET/CT in this patient with PSA of 0.3 ng/mL. This finding corresponds to the lowest PSA value associated with a positive FCH PET/CT result in this study. Note that the left ureter (thin arrow) is laterally adjacent to this lymph node. Because FCH is excreted into the urine, careful anatomical correspondence with CT images is necessary to avoid a misinterpretation due to urinary artifacts. Gentle fluid restriction was also used in this patient to reduce the amount of ureteral activity at the time of PET scanning. b At follow-up, this lymph node was found to be enlarged, measuring 13 mm on diagnostic CT performed with oral and intravenous contrast after the PSA level had risen to 1.0 ng/mL in 1 month. Additional lymph nodes measuring up to 6 mm (not shown) were also found along the left retroperitoneum at follow-up
Two patients had bone scintigraphic findings consistent with metastatic disease. Four additional patients had bone scintigraphic studies classified as unremarkable (n = 2) or non-specific (n = 2), but were noted to have areas of abnormal skeletal FCH uptake on corresponding PET/CT. Radiographic follow-up in all cases, along with biopsy in one of the cases associated with a non-specific bone scan finding (Fig. 4), confirmed the FCH PET/CT findings to be metastatic disease. Specific patterns of abnormal findings on FCH PET/CT for each treatment category are summarized in Table 2.
Metastatic prostate cancer response to androgen deprivation. a Bone scintigraphy shows a solitary abnormal finding in the left ischium (white arrow). This finding was deemed equivocal in the absence of any other scintigraphic findings suggestive of metastatic disease. b FCH PET demonstrated a corresponding focus of increased uptake in the same region (arrow) with a maximum SUV of 5.5. c On PET/CT, sclerotic changes in the ischium are evident around the area of increased PET activity. As was the case with bone scintigraphy, there was no other evidence of metastasis on CT. d MRI directed by the PET findings demonstrated a corresponding 1-cm diameter marrow-based lesion in the left ischium (arrow). A CT-guided biopsy of this lesion confirmed it to be metastatic prostate cancer. e A second follow-up PET/CT scan following hormonal therapy shows normalization of FCH uptake in the left ischium. This change in FCH uptake coincided with a fall in the PSA level to 0.1 ng/mL. f A persistent sclerotic area in the left ischium can be seen on CT, although the abnormal activity on PET did resolve in concert with the PSA response
Discussion
Clinical outcomes for patients with recurrent prostate cancer could improve further if salvage treatments were individualized based on accurate delineation of cancer recurrence. Unfortunately, the localization of recurrent prostate cancer remains a significant diagnostic challenge, particularly at low PSA levels. In this study, it proved possible to identify local, lymphatic, and hematogenous routes of metastatic disease progression on the basis of whole-body FCH PET/CT with tumor detection rates directly correlated to PSA levels at the time of imaging.
Such a relationship between PSA level and the detection of prostate cancer by PET was first reported in studies using the tracer 11C-labeled choline. In one study, 11C-choline PET correctly detected tumor recurrence in 78 % of patients after external beam RT and 38 % of patients after RP, although there were no positive scan results at a PSA level below 5 ng/mL [12]. Another study which focused on post-RP patients with biopsy-confirmed tumor recurrences reported a more favorable diagnostic sensitivity of 89 % and positive predictive value of 72 % for 11C-choline PET/CT detection of recurrent prostate cancer at PSA <2.5 ng/mL [19]. A direct relationship between positive findings on 11C-choline PET/CT and PSA level in patients with recurrent prostate cancer was suggested in one study, with detection rates of 36, 43, and 62 % noted at PSA values of <1, 1 to <2, and 2 to <3 ng/mL, respectively [13]. Higher detection rates with 11C-choline PET/CT have also been associated with higher PSA velocities or shorter doubling times [20].
The potential relationship between PSA level and tumor detection using fluorine-18 labeled choline analogs has also been investigated. Despite potential advantages to imaging with a fluorine-18 labeled PET tracer, findings from two studies also suggest that FCH PET/CT sensitivity diminishes as the PSA level approaches the 1–4 ng/mL range [9, 21]. Another recent study of FCH PET/CT estimated the diagnostic sensitivity in post-prostatectomy patients to be 20, 44, and 81.8 % at PSA levels of ≤1, >1 and ≤5, and >5 ng/mL, respectively [22]. These previous studies are in accord with the current study. Notably, the current study was drawn from a predominantly Asian population and 32/50 of the patients enrolled were of Asian ancestry. In contrast, the previous studies were conducted in Europe at institutions where the clinical use of FCH was pioneered. The current study therefore serves to generalize some of the performance characteristics of FCH PET/CT to a population of prostate cancer patients that may differ from Western patients, particularly with regard to responsiveness to hormonal therapy and rates of disease progression [23, 24].
Sub-optimal detection rates at lower PSA values could potentially limit the value of FCH PET/CT in guiding treatment decisions for prostate cancer salvage therapy. This is because disease control rates for salvage treatments such as external beam radiation for recurrent prostate cancer following RP are also known to vary as a function of PSA level. For example, 6-year progression-free survival for salvage radiotherapy in patients with PSA levels of 0.5, 0.51–1.0, 1.01–1.5, and >1.5 ng/mL have been estimated at of 48, 40, 28, and 18 %, respectively [25]. Thus, the PSA range associated with ideal detection rates for choline-based PET/CT may fall above the critical range of PSA values, where salvage therapy would provide the greatest clinical benefit. However, since detection failures with PET could be related to low tumor volume, there should be some prognostic relevance associated with a negative result on FCH PET/CT. Prognostic information gleaned from FCH PET/CT could therefore potentially aid in decisions on the timing of aggressive treatments. Further research is needed to determine the value of FCH PET/CT as a prognostic tool in patients with recurrent prostate cancer.
As a potential limitation, this study employed more than one method for confirming abnormal PET/CT findings, since biopsy confirmation was not always feasible to pursue in the clinical setting. Longitudinal follow-up and imaging were therefore necessary when biopsy data were unavailable for correlation with FCH PET/CT findings. The use of several methods as the standard of reference is not unusual for studies evaluating diagnostic imaging tests for recurrent prostate cancer due to the limited availability of tissue biopsy confirmations of metastatic disease in these patients.
In this study, visual analyses served to classify PET scans as either positive or negative for tumor recurrence, introducing potential subjectivity in PET/CT interpretations. An alternative approach would have been to apply a semi-quantitative threshold such as SUV as a discriminator of malignant involvement. Although a tumor-to-background ratio >2 correctly classified all abnormal lesions in this study, diagnostic thresholds based on SUV have not yet been validated for FCH PET/CT, and such criteria could vary depending on the imaging protocol being used [26]. Fortunately, many anatomic regions predisposed to metastatic involvement by prostate cancer, such as the bone marrow and pelvic lymphatics, exhibit relatively low FCH uptake under normal conditions, making these areas amenable to visual inspection. Furthermore, since all patients underwent primary treatments for prostate cancer, focal increases in activity within the prostatic region could be considered abnormal for the purpose of this study. A decade of mutual experience in FCH PET and PET/CT likely underlies the strong concordance between the two image interpreters in this study. However, as with any PET tracer, all readers must be aware of the normal tracer biological distribution and normal variants in uptake for image interpretations to be reproducible. Although no false-positive results were encountered in this study, there are several issues known to limit the diagnostic specificity of FCH PET/CT [27]. First, is non-specific increased uptake by benign lymph nodes, particularly those in the axillary, mediastinal/hilar, and inguinal regions [27, 28]. Another issue which may lead to interpretive pitfalls is the excretion of intact radiopharmaceutical by the renal system. As illustrated in Fig. 3, careful study of retroperitoneal and pelvic anatomy with anatomical CT correspondence is essential for accurately assessing pathologic findings in prostate cancer.
Treatments for recurrent prostate cancer could benefit from further individualization based on the mode of tumor recurrence depicted by imaging. For example, localized RT may be guided to lesions that may lead to pathologic fracture or urinary obstruction, thus reducing the risk of metastatic complications. By differentiating the sites of tumor recurrence, imaging may also help in selecting patients who may benefit from novel drugs that target a specific mechanism of metastatic progression. For example, a patient found to have bone-dominant metastatic disease may be steered toward treatments that can specifically interfere with the osteomimetic properties of prostate cancer. Estimations of tumor burden in the later stages of prostate cancer may also prove useful for planning palliative options. Thus, there are multiple opportunities for integrating FCH PET/CT into the clinical management of recurrent prostate cancer.
Conclusion
FCH PET/CT may facilitate the single-step whole-body assessment of metastatic recurrence and tumor progression in prostate cancer patients with rising PSA levels after primary therapy. This technique has shown the ability to assess disease progression along all known routes of prostate cancer recurrence (local, lymphatic and hematogenous), although the likelihood of tumor detection may be related to the PSA level.
References
Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003;169:517–23.
Chism DB, Hanlon AL, Horwitz EM, Feigenberg SJ, Pollack A. A comparison of the single and double factor high-risk models for risk assignment of prostate cancer treated with 3D conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:380–5.
Hricak H, Schoder H, Pucar D, Lis E, Eberhardt SC, Onyebuchi CN, et al. Advances in imaging in the postoperative patient with a rising prostate-specific antigen level. Semin Oncol. 2003;30:616–34.
Wilkinson S, Chodak G. The role of 111indium-capromab pendetide imaging for assessing biochemical failure after radical prostatectomy. J Urol. 2004;172:133–6.
Cher ML, Bianco FJ Jr, Lam JS, Davis LP, Grignon DJ, Sakr WA, et al. Limited role of radionuclide bone scintigraphy in patients with prostate specific antigen elevations after radical prostatectomy. J Urol. 1998;160:1387–91.
Stone NN, Stock RG, White I, Unger P. Patterns of local failure following prostate brachytherapy. J Urol. 2007;177:1759–63 (discussion 63–4).
Swanson GP, Hussey MA, Tangen CM, Chin J, Messing E, Canby-Hagino E, et al. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol. 2007;25:2225–9.
Heinisch M, Dirisamer A, Loidl W, Stoiber F, Gruy B, Haim S, et al. Positron emission tomography/computed tomography with F-18-fluorocholine for restaging of prostate cancer patients: meaningful at PSA < 5 ng/ml? Mol Imaging Biol. 2006;8:43–8.
Cimitan M, Bortolus R, Morassut S, Canzonieri V, Garbeglio A, Baresic T, et al. [(18)F]fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur J Nucl Med Mol Imaging. 2006;33:1387–98.
Vees H, Buchegger F, Albrecht S, Khan H, Husarik D, Zaidi H, et al. 18F-choline and/or 11C-acetate positron emission tomography: detection of residual or progressive subclinical disease at very low prostate-specific antigen values (<1 ng/mL) after radical prostatectomy. BJU Int. 2007;99:1415–20.
Steiner C, Vees H, Zaidi H, Wissmeyer M, Berrebi O, Kossovsky MP, et al. Three-phase (18)F-fluorocholine PET/CT in the evaluation of prostate cancer recurrence. Nuklearmedizin. 2009;48:1–9.
de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ. 11C-Choline positron emission tomography for the evaluation after treatment of localized prostate cancer. Eur Urol. 2003;44:32–8 (discussion 8–9).
Krause BJ, Souvatzoglou M, Tuncel M, Herrmann K, Buck AK, Praus C, et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:18–23.
Glunde K, Jie C, Bhujwalla ZM. Molecular causes of the aberrant choline phospholipid metabolism in breast cancer. Cancer Res. 2004;64:4270–6.
Ramirez de Molina A, Rodriguez-Gonzalez A, Gutierrez R, Martinez-Pineiro L, Sanchez J, Bonilla F, et al. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun. 2002;296:580–3.
DeGrado TR, Baldwin SW, Wang S, Orr MD, Liao RP, Friedman HS, et al. Synthesis and evaluation of (18)F-labeled choline analogs as oncologic PET tracers. J Nucl Med. 2001;42:1805–14.
Bansal A, Shuyan W, Hara T, Harris RA, Degrado TR. Biodisposition and metabolism of [(18)F]fluorocholine in 9L glioma cells and 9L glioma-bearing fisher rats. Eur J Nucl Med Mol Imaging. 2008;35:1192–203.
Lim J, Dorman E, Cabral C. Automated production of [18F]FECh and [18F]FCH: preparation and use of [18F]fluoroalkane sulfonates and fluoroalkylation agents. J Label Compd Radiopharm. 2003;46:S46.
Rinnab L, Simon J, Hautmann RE, Cronauer MV, Hohl K, Buck AK, et al. [(11)C]choline PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy. World J Urol. 2009;27:619–25.
Castellucci P, Fuccio C, Nanni C, Santi I, Rizzello A, Lodi F, et al. Influence of trigger PSA and PSA kinetics on 11C-choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. J Nucl Med. 2009;50:1394–400.
Husarik DB, Miralbell R, Dubs M, John H, Giger OT, Gelet A, et al. Evaluation of [(18)F]-choline PET/CT for staging and restaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:253–63.
Pelosi E, Arena V, Skanjeti A, Pirro V, Douroukas A, Pupi A, et al. Role of whole-body 18F-choline PET/CT in disease detection in patients with biochemical relapse after radical treatment for prostate cancer. Radiol Med. 2008;113:895–904.
Namiki M, Akaza H, Lee SE, Song JM, Umbas R, Zhou L, et al. Prostate Cancer Working Group report. Jpn J Clin Oncol. 2010;40(Suppl 1):i70–5.
Fukagai T, Namiki TS, Carlile RG, Yoshida H, Namiki M. Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU Int. 2006;97:1190–3.
Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41.
Kwee SA, Wei H, Sesterhenn I, Yun D, Coel MN. Localization of primary prostate cancer with dual-phase 18F-fluorocholine PET. J Nucl Med. 2006;47:262–9.
Schillaci O, Calabria F, Tavolozza M, Ciccio C, Carlani M, Caracciolo CR, et al. 18F-choline PET/CT physiological distribution and pitfalls in image interpretation: experience in 80 patients with prostate cancer. Nucl Med Commun. 2010;31:39–45.
Rietbergen DD, van der Hiel B, Vogel W, Stokkel MP. Mediastinal lymph node uptake in patients with prostate carcinoma on F18-choline PET/CT. Nucl Med Commun. 2011;32:1143–7.
Acknowledgments
This work was supported by United States National Institutes of Health National Cancer Institute Grant R21CA139687.
Conflict of interest
The authors declare that they have no competing interests, financial or otherwise, that might be perceived to influence the results and/or discussion reported in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kwee, S.A., Coel, M.N. & Lim, J. Detection of recurrent prostate cancer with 18F-fluorocholine PET/CT in relation to PSA level at the time of imaging. Ann Nucl Med 26, 501–507 (2012). https://doi.org/10.1007/s12149-012-0601-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-012-0601-8