Abstract
Objective
Adenosine is an endogenous modulator of synaptic functions in the central nervous system. The effects of adenosine are mediated by at least four adenosine receptor subtypes. Decreased density of adenosine A1 receptors, which is a major subtype adenosine receptor in the hippocampus, has been reported in vitro in Alzheimer’s disease. We evaluated adenosine A1 receptor in the brain of elderly normal subjects and patients with Alzheimer’s disease (n = 8 and 6, respectively), using positron emission tomography (PET) and 8- dicyclopropylmethyl-1-[11C]methyl-3-propylxanthine ([11C]MPDX).
Methods
A 60-min PET scan with [11C]MPDX was performed. The patients with Alzheimer’s disease also underwent PET with [18F]fluorodeoxyglucose (FDG). The binding potential of [11C]MPDX was quantitatively calculated in the regions of interest (ROIs) placed on the frontal, medial frontal, temporal, medial temporal, parietal, and occipital cortices, striatum, thalamus, cerebellum, and pons. Statistical parametric mapping (SPM2) was used for analysis of [11C]MPDX and FDG-PET.
Results
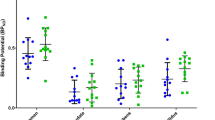
In the ROI-based analysis, the binding potential of [11C]MPDX in patients with Alzheimer’s disease was significantly lower in the temporal and medial temporal cortices and thalamus than that in elderly normal subjects (P = 0.038, 0.028, and 0.039, respectively). SPM analysis also showed significant decreased binding potential in the temporal and medial temporal cortices and thalamus in patients with Alzheimer’s disease. FDG uptake was significantly decreased in the temporoparietal cortex and posterior cingulate gyrus.
Conclusions
Decreased binding of [11C]MPDX in patients with Alzheimer’s disease was detected in temporal and medial temporal cortices and thalamus. This pattern possibly differed from the hypometabolism pattern of FDG. [11C]MPDX PET is valuable for the detection of degeneration in the temporal and medial temporal cortices and corticothalamic transmission, and may provide a different diagnostic tool from FDG-PET in brain disorders such as Alzheimer’s disease.
Similar content being viewed by others
References
Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Ann Rev Neurosci 2001;24:31–55.
Yacoubi ME, Costentin J, Vaugeois JM. Adenosine A2A receptors and depression. Neurology 2003;61:S82–S87.
von Lubitz DK. Adenosine in the treatment of stroke: yes, maybe, or absolutely not? Expert Opin Investig Drugs 2001;10:619–632.
Haas HL, Selbach O. Functions of neuronal adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 2000;362:375–381.
Collis MG, Hourani SMO. Adenosine receptor subtypes. Trends Pharmacol Sci 1993;14:360–366.
Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, et al. Nomenclature and classification of purinoceptors. Pharmacol Rev 1994;46:143–156.
Corradetti R, Lo Conte G, Moroni F, Passani MB, Pepeu G. Adenosine decreases aspartate and glutamate release from rat hippocampal slices. Eur J Pharmacol 1984;104:19–26.
Dolphin AC, Archer ER. An adenosine agonist inhibits and a cyclic AMP analogue enhances the release of glutamate but not GABA from slices of rat dentate gyrus. Neurosci Lett 1983;43:49–54.
Dunwiddie TV. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol 1985;27:63–139.
Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release? Trends Pharmacol Sci 1988;9:130–134.
Fredholm BB, Hedqvist P. Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol 1980;29:1635–1643.
Phillis JW, Wu PH. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol 1981;16:187–239.
Schoenberg BS. Epidemiology of Alzheimer’s disease and other dementing disorders. J Chronic Dis 1986;39:1095–1104.
Fratiglioni L, Grut M, Forsell Y, Viitanen M, Grafstrom M, Holmen K, et al. Prevalence of Alzheimer’s disease and other dementias in an elderly urban population: relationship with age, sex, and education. Neurology 1991;41:1886–1892.
Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health 1998;88:1337–1342.
Weinberger M, Gold DT, Divine GW, Cowper PA, Hodgson LG, Schreiner PJ, et al. Expenditures in caring for patients with dementia who live at home. Am J Public Health 1993;83:338–341.
Osttbye T, Crosse E. Net economic costs of dementia in Canada. CMAJ 1994;151:1457–1463.
Ernst R, Hay JW, Fenn C, Tinklenberg J, Yesavage JA. Cognitive function and the costs of Alzheimer’s disease. Arch Neurol 1997;54:687–693.
Jansen K, Faull RLM, Dragunow M, Synek BJL. Alzheimer’s disease: changes in hippocampal N-methyl-d-aspartate, quisqualate, neurotensin, adenosine, benzodiazepine, serotonin and opioid receptors: an autoradiographic study. Neuroscience 1990;39:613–627.
Ulas J, Brunner LC, Nguyen L, Cotman CW. Reduced density of adenosine A1 receptors and preserved coupling of adenosine A1 receptors to G proteins in Alzheimer hippocampus: a quantitative autoradiographic study. Neuroscience 1993;52:843–854.
Jaarsma D, Sebens B, Korf J. Reduction of adenosine A1- receptors in the perforant pathway terminal zone in Alzheimer hippocampus. Neurosci Lett 1991;121:111–114.
Kalaria RN, Sromek S, Wilcox BJ, Unnerstall JR. Hippocampal adenosine A1 receptors are decreased in Alzheimer’s disease. Neurosci Lett 1990;118:257–260.
Ishii K, Sasaki H, Kono AK, Miyamoto N, Fukuda T, Mori E. Comparison of gray matter and metabolic reduction in mild Alzheimer’s disease using FDG-PET and voxel-based morphometric MR studies. Eur J Nucl Med 2005;32:959–963.
Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med 2005;32:486–510.
Meguro K, LeMestric C, Landeau B, Desgranges B, Eustache F, Baron JC. Relations between hypometabolism in the posterior association neocortex and hippocampal atrophy in Alzheimer’s disease: a PET/MRI correlative study. J Neurol Neurosurg Psychiatry 2001;71:315–321.
Fukumitsu N, Ishii K, Kimura Y, Oda K, Sasaki T, Mori Y, et al. Imaging of adenosine A1 receptors in the human brain by positron emission tomography with [11C]MPDX. Ann Nucl Med 2003;17:511–515.
Fukumitsu N, Ishii K, Kimura Y, Oda K, Sasaki T, Mori Y, et al. Adenosine A1 receptor mapping of the human brain by PET with 8-dicyclopropylmethyl-1-11C-methyl-3- propylxanthine. J Nucl Med 2005;46:32–37.
Kimura Y, Ishii K, Fukumitsu N, Oda K, Sasaki T, Kawamura K, et al. Quantitative analysis of adenosine A1 receptors in human brain using positron emission tomography and [1-methyl-11C]8-dicyclopropylmethyl-1-methyl-3-propylxanthine. Nucl Med Biol 2004;31:975–981.
Naganawa M, Kimura Y, Nariai T, Ishii K, Oda K, Manabe Y, et al. Omission of serial arterial blood sampling in neuro-receptor imaging with independent component analysis. Neuroimage 2005;26:885–890.
Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361–367.
Noguchi J, Ishiwata K, Furuta R, Simada J, Kiyosawa M, Ishii S, et al. Evaluation of carbon-11 labeled KF15372 and its ethyl and methyl derivatives as a potential CNS adenosine A1 receptor ligand. Nucl Med Biol 1997;24:53–59.
Ishiwata K, Nariai T, Kimura Y, Oda K, Kawamura K, Ishii K, et al. Preclinical studies on [11C]MPDX for mapping adenosine A1 receptors by positron emission tomography. Ann Nucl Med 2002;16:377–382.
Ardekani BA, Braun M, Hutton BF, Kanno I, Iida H. A fully automatic multimodality image registration algorithm. J Comput Assist Tomog 1995;19:615–623.
Dragunow M, Murphy K, Leslie RA, Robertson HA. Localization of adenosine A1-receptors to the terminals of the perforant path. Brain Res 1988;462:252–257.
Hyman BT, Van Hoesen GW, Kromer LJ, Damasio AR. Perforant pathway changes and the memory impairment of Alzheimer’s disease. Ann Neurol 1986;20:472–481.
Stephen RE, Janke AL, Chark JB. Gray and white matter changes in Alzheimer’s disease: a diffusion tensor imaging study. J Magn Reson Imaging 2008;27:20–26.
Medina D, DeToledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, et al. White matter changes in mild cognitive impairment and AD: a diffusion tensor imaging study. Neurobiol Aging 2006;27:663–672.
Zahn R, Juengling F, Bubrowski P, Jost E, Dykierek P, Talazko J, et al. Hemisphere asymmetries of hypometabolism associated with semantic memory impairment in Alzheimer’s disease: a study using positron emission tomography with fluorodeoxygulcose-F18. Psychiatry Res Neuroimaging 2004;132:159–172.
Fastbom J, Pazos A, Probst A, Palacios JM. Adenosine A1 receptors in the human brain: a quantitative autoradiographic study. Neuroscience 1987;22:827–839.
Sakamoto S, Ishii K, Sasaki M, Hosaka M, Mori T, Matsui M, et al. Differences in cerebral metabolic impairment between early and late onset types of Alzheimer’s disease. J Neurol Sci 2002;15:27–32.
Kim EJ, Cho SS, Jeong Y, Park KC, Kang E, Kim SE, et al. Glucose metabolism in early onset versus late onset Alzheimer’s disease: an SPM analysis of 120 patients. Brain 2005;128:1790–1801.
Ibanez V, Pietrini P, Alexander GE, Furey ML, Teichberg D, Rajapakse JC, et al. Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease. Neurology 1998;50:1585–1593.
Mosconi L, Sorbi S, de Leon MJ, Li Y, Nacmias B, Myoung PS, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med 2006;47:1778–1786.
Chetelat G, Desgranges B, Landeau B, Mezenge F, Poline JB, de la Sayette V, et al. Direct voxel-based comparison between grey matter hypometabolism and atrophy in Alzheimer’s disease. Brain 2008;131:60–71.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukumitsu, N., Ishii, K., Kimura, Y. et al. Adenosine A1 receptors using 8-dicyclopropylmethyl-1-[11C]methyl-3-propylxanthine PET in Alzheimer’s disease. Ann Nucl Med 22, 841–847 (2008). https://doi.org/10.1007/s12149-008-0185-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-008-0185-5