Abstract
Negative symptoms in schizophrenia remain a clinical challenge with small effect sizes and evidence for pharmacological or psychotherapeutic treatment approaches. Studies suggest that electroconvulsive therapy (ECT) holds some promise as a treatment option of often persistent negative symptoms with clinically meaningful effects. This review summarizes the existing evidence on the efficacy of ECT on negative symptoms in patients with schizophrenia. Thirty-five publications were included in this literature review comprising 21 studies, two meta-analyses, eight reviews and four case reports. Conclusions should be interpreted cautiously, given the small number and methodological shortcomings of the included publications with a variation of study designs and missing standardized protocols. Implications for future research and practice are critically discussed. Recommendations are given to provide more evidence that will meet the clinical challenge of reducing the negative symptoms in schizophrenia. Study designs that focus explicitly on negative symptoms and assess patients over longer follow up periods could be helpful. Future research should include control groups, and possibly establish international multicentered studies to get a sufficient study population. Findings suggest that patients with schizophrenia resistant to pharmacological treatment might benefit from ECT. A risk and benefit assessment speaks in favour of the ECT treatment. Future practice of ECT should include a combination treatment with antipsychotics. Whereas the use of anaesthetics and electrode placement does not seem to play a role, the recommendation regarding frequency of ECT treatments is currently three times a week, For the assessment of negative symptoms the assessment tool should be chosen carefully.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Electroconvulsive therapy (ECT) was introduced by Cerletti and Bini in 1938 as a therapy for schizophrenia and is today mainly used in severe major depression (Cerletti & Bini, 1938; Chanpattana & Andrade, 2006; Chanpattana & Chakrabhand, 2001a; Fink, 1994; Moeller et al., 2017). ECT is a so-called somatic treatment that uses electrical stimulation to induce seizures while patients receive anaesthesia and muscle relaxant (Canadian Agency for Drugs and Technologies in Health, 2014; Ding & White, 2002; Ipekcioglu et al., 2018). The use of ECT for schizophrenia declined after the emergence of effective pharmacological treatment for positive symptoms during the 1960s and 1970s (Chanpattana & Andrade, 2006; Fink & Sackeim, 1996). However, interest in ECT as a treatment for schizophrenia re-emerged as the limitations in the efficacy of antipsychotic drugs towards various symptom domains of schizophrenia were increasingly recognized over time (Chanpattana & Andrade, 2006; Fink & Sackeim, 1996). Although some antipsychotics (e.g., clozapine) have been specifically approved for the treatment of resistant schizophrenia, given a poor treatment adherence in patients with resistant schizophrenia the risk of a psychotic relapse increases. This may further lead to impaired social and cognitive functioning, psychiatric hospitalizations and increased treatment costs. Additionally clozapine adverse effects need to be carefully monitored by clinicians (De Berardis et al., 2018). This emphasizes the need of alternative treatment options. Nonetheless, no specific treatment can be conclusively recommended for addressing negative symptoms especially in treatment resistant schizophrenia (Remington et al., 2016).
Negative symptoms are one the most important of the six core symptoms of schizophrenia according to the new ICD-11 (i.e., negative, positive, depressive, manic, psychomotor and cognitive symptoms) (Keeley & Gaebel, 2018) and there is no recommended treatment with lasting effects against negative symptoms (Marder & Galderisi, 2017; Remington et al., 2016). Negative symptoms are associated with a high burden due to their negative impact on social functioning, subjective quality of life, and physical health risks (Marder & Galderisi, 2017). Five key sub-domains of negative symptoms have been identified: (1) avolition, (2) anhedonia, (3) blunted affectivity, (4) reduced social behaviour and (5) alogia (Foussias et al., 2014; Kirkpatrick et al., 2006; Marder & Galderisi, 2017; Remington et al., 2016). Interest in negative symptoms has increased rapidly over the last several decades, given its large share of the burden of disease in schizophrenia (Remington et al., 2016). A further distinction between primary (i.e. deficit) and secondary negative symptoms came into the focus of research undertaken during the 1980s (Remington et al., 2016). Efforts continue to conceptualize and measure negative symptoms to distinguish their impact from other symptom domains and establish protocols for evidence-based treatments (Remington et al., 2016). From a treatment perspective, according to the MATRICS consensus statement, the distinction between primary and secondary negative symptoms is not essential regarding persistent negative symptoms (Kirkpatrick et al., 2006; Remington et al., 2016). Primary negative symptoms have been identified in approximately 25% to 30% of individuals with chronic schizophrenia, according to some studies (Kirkpatrick et al., 2001; Remington et al., 2016). It has also been suggested that a functional and social recovery occurs in less than 15% of individuals with schizophrenia and that persisting negative symptoms contribute towards problems experienced in daily life (Austin et al., 2013; Remington et al., 2016). Furthermore, stigma and discrimination are worldwide linked to this condition. In particular, the increasing explanation of schizophrenia as a genetic disorder influences the perception of other people’s beliefs about dangerousness and unpredictability and people’s desire for social distance. So, this may potentially enhance stigmatizing attitudes but also social withdrawal of patients especially those with a high burden of negative symptoms and less stigma resistance (Campellone et al., 2014; Serafini et al., 2011).
Several promising interventions for negative symptoms in schizophrenia have been investigated, including pharmacological treatment approaches, brain stimulation and non-somatic approaches (e.g. cognitive behavioral therapy) (Remington et al., 2016). ECT is often used for patients affected by schizophrenia with a treatment focus on positive and depressive symptoms (Chanpattana & Andrade, 2006; Hasan et al., 2015a) and the American Psychiatric Association (APA) guidelines recommend ECT for patients with severe psychotic symptoms, catatonia and suicidality (Hasan et al., 2015b; Lehman et al., 2004). There have also been a few related studies published since the 1960s (Fink & Sackeim, 1996; Lehnhardt et al., 2012), but the first study explicitly investigating the effectiveness and response predictors for ECT in patients with predominantly negative symptoms was recently published in 2014 (Pawełczyk et al., 2014a). Although there have been clinical studies since the 1960s and randomized control studies in the 1970s (Fink & Sackeim, 1996; Lehnhardt et al., 2012), the state of research concerning ECT for schizophrenia and predominantly negative symptoms is far less researched than ECT treatment for affective disorders (Lehnhardt et al., 2012; Tharyan & Adams, 2005; The UK, 2003).
As described earlier, the negative symptoms of schizophrenia are a very limiting and distressing group of symptoms for patients (Marder & Galderisi, 2017). Despite the aforementioned research into pharmacological and non-somatic treatment options as well as treatment options using brain stimulation, there is still no resounding success in the treatment of these symptoms (Remington et al., 2016). With no new breakthrough treatment options in sight in the near future, it is even more important to focus on the tools currently available and to get the best out of them for the treatment of patients (Remington et al., 2016). This includes, in particular, ECT treatment, for which there are not yet sufficient prefabricated treatment plans in this area.
Therefore, this literature review summarizes the latest literature on the utilization of ECT for patients affected by schizophrenia, focusing on findings related to negative symptoms. The review specifically aims to identify gaps in the existing literature and to highlight related trends (e.g. research methods used, research regions and treatment plans). Practitioners will be informed about how ECT is used in the treatment of negative symptoms. The review aims to provide information that can be used for the development of future and much needed unified ECT treatment plans for negative symptoms experienced by people with schizophrenia. Since there is still a great need for treatment options for negative symptoms, it is more than necessary to exploit the possibilities of available therapies. In this case the coordination of study designs for the comparability of research results would be a necessary next step.
The paper next outlines the methodology including the search strategy and evaluation process for identifying papers to be included in the review. The results summarizing the output of the different included papers with a focus on the effects of ECT treatment on negative symptoms will be given. In the discussion section the different aspects of the papers will be summarized and discussed for future research and practice.
Methods
The present literature review was carried out to (1) summarize the latest literature on the utilization of ECT for patients affected by schizophrenia with a focus on findings related to negative symptoms, (2) to identify potential research needs in the area, and (3) to offer recommendations for future research. Some steps of our process, including devising a method of locating relevant knowledge, defining eligibility criteria for screening, establishing procedures for abstracting, summarizing and synthesizing the identified knowledge, and sharing process figures and search-related resources archived in the appendix, are more associated with systematic reviews (Dijkers, 2009). Nonetheless, these steps were merely followed to enhance completeness and promote transparency in our coverage of existing knowledge and to organize teamwork (Powell et al., 2014). For example, no effort was expended to assess and exclude studies based on methodological quality or appropriateness, as this was outside the scope of this literature review.
Search Strategy
Searches were performed to find studies using electronic bibliographic databases, reference-list searching and recommendations from field experts. The respective electronic bibliographic databases included: MEDLINE (PubMed), CINAHL (EBSCOHost), PsychInfo (ProQuest) and Scopus. Search terms were broadly based on concepts relating to electroconvulsive therapy, schizophrenia and negative symptoms (see Appendix Table 2). Publications mentioning at least one keyword from all three search concepts in their titles and abstracts were returned. This decision was made to improve the search’s effectiveness and efficiency. It was more likely that publications mentioning these three concepts across the title or abstract would be more relevant than publications that did not. For example, our preliminary searches revealed that studies mentioning keywords for negative symptoms in the publication’s full text (e.g., Positive and Negative Syndrome Scale (PANSS)) are often not eligible and return almost five times the number of results. The reference lists of included publications were also searched to avoid missing relevant publications not identified during the database search. Several field experts were also familiarized with the aims of the study and asked to share papers that might meet the eligibility criteria for considered literature.
Eligibility Criteria and Assessment
Included papers focus on the utilization of ECT for patients affected by schizophrenia, focusing on findings related to negative symptoms. Studies published from 2000 onwards were chosen considering that brief pulse ECT was widely adopted during this time and replaced sinus wave ECT usage in previous times (Ward et al., 2018). The studies were required to be published in the English or German language. Original articles with interventional studies, retrospective studies, naturalistic observation studies, naturalistic comparison studies, as well as case reports, literature reviews, systematic reviews and meta-analysis were considered for inclusion in this review. Publications in the form of commentaries, letters to the editors, and editorials were excluded.
Title, abstract and full-text screening was conducted independently by two researchers to determine eligibility. This approach involving comprehensive and total second screening was made to increase screenings’ reliability and reduce any possible reviewer bias (McDonagh et al., 2013). Any inconsistencies in screening decisions were later discussed and resolved.
Data Extraction and Synthesis of Results
The following data were extracted after identifying relevant publications: author, year, patient demographic information (e.g., gender and age), study design, number of participants, symptoms, treatment plans including ECT treatment and types of additional antipsychotics and dosage, clinical interview type and study results. The publications were first categorized based on the outcome of ECT on negative symptoms of the patients with schizophrenia and then summarized based on the emergent categories.
Results
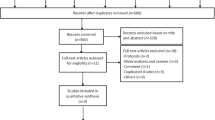
This review included 35 publications on the utilization of ECT for patients affected by schizophrenia, focusing on findings related to negative symptoms (see Table 1). Figure 1. illustrates the flowchart of the review identification and selection process.
Study Characteristics and Design
From the 35 included publications investigating the effect of ECT on schizophrenia, 16 were prospective interventional studies (Chanpattana & Chakrabhand, 2001a; Chanpattana & Kramer, 2003; Chanpattana & Sackeim, 2010; Ipekcioglu et al., 2018; Jia et al., 2019; Kho et al., 2004; Li et al., 2016; Masoudzadeh et al., 2007; Pawełczyk et al., 2014a, 2014b; Petrides et al., 2015; Tang & Ungvari, 2001, 2002, 2003; Üçok & Çakir, 2006; Zhang et al., 2012), two naturalistic observation studies (Kim et al., 2018; Usta Saglam et al., 2020), one naturalistic comparison study (Bansod et al., 2018), one retrospective controlled study (Jia et al., 2019) and one retrospective chart review (Xiang et al., 2015). Six literature reviews (Chanpattana, 2007; Chanpattana & Andrade, 2006; Dokucu, 2015; Hasan et al., 2015b; Lehnhardt et al., 2012; Remington et al., 2016), two systematic reviews (Sinclair et al., 2019; Tharyan & Adams, 2005) and two meta-analyses (Ahmed et al., 2017; Zheng et al., 2016), giving an overview of some aspects of the effect of ECT on schizophrenia, were also included in this review. The review also included four case reports showing individual effects on negative symptoms following ECT (Martinotti et al., 2011; Moeller et al., 2017; Park & Lee, 2014; Seethalakshmi et al., 2006).
In two of the studies, the assessors were blinded (Davarinejad et al., 2019; Petrides et al., 2015) and only one study was double-blinded with sham treatment (Masoudzadeh et al., 2007). Of the prospective interventional studies, 15 were considered open-label studies as they did not mention blinding (Bansod et al., 2018; Chanpattana & Chakrabhand, 2001a; Chanpattana & Kramer, 2003; Chanpattana & Sackeim, 2010; Ipekcioglu et al., 2018; Kho et al., 2004; Kim et al., 2018; Li et al., 2016; Pawełczyk et al., 2014a, 2014b; Tang & Ungvari, 2001, 2002, 2003; Üçok & Çakir, 2006; Zhang et al., 2012). Five of these prospective interventional studies also used a controlled study design (Li et al., 2016; Petrides et al., 2015; Tang & Ungvari, 2002; Üçok & Çakir, 2006; Zhang et al., 2012), but these studies were limited by control groups with patients who refused ECT and instead only received pharmacotherapy. The only retrospective study during the period from 2000 to 2021 was a controlled study comparing ECT to electroacupuncture and a control group (Jia et al., 2019).
Sample Characteristics
Participants in included studies were diagnosed with schizophrenia according to DSM-IV, DSM-V (American Psychiatric Association, 2013) or ICD-10 criteria, and the inclusion criteria differed between the studies and were defined differently by each of the authors. In 15 of the included 21 original articles, the samples consisted of patients nonresponsive to antipsychotic medication or suffered from so-called treatment-resistant schizophrenia (TRS) (Lehman et al., 2004; Petrides et al., 2015). Diagnostic criteria for treatment resistance varied between studies. Only 3 of the 21 studies focused on patients with first-episode psychosis (Bansod et al., 2018; Üçok & Çakir, 2006; Zhang et al., 2012). Two publications (Pawełczyk et al., 2014a, 2014b) focused on the effectiveness of ECT on TRS patients with dominant negative symptoms. Other inclusion criteria included illness length of at least two (Chanpattana & Chakrabhand, 2001a; Chanpattana & Kramer, 2003; Chanpattana & Sackeim, 2010; Davarinejad et al., 2019; Petrides et al., 2015) or three years (Tang & Ungvari, 2002, 2003), a minimum score of 37 points at the Brief Psychiatric Rating Scale (BPRS) (Chanpattana & Chakrabhand, 2001a; Chanpattana & Kramer, 2003; Chanpattana & Sackeim, 2010), at least six months of continuous hospitalization, ability and willingness to give informed consent (Tang & Ungvari, 2002, 2003) or history of previous ECT response (Ipekcioglu et al., 2018). Exclusion criteria were less heterogeneous between the studies, with eleven of them excluding patients with a severe medical disorder in which ECT is contra-indicated or current use of alcohol or substance abuse (Chanpattana & Chakrabhand, 2001a; Chanpattana & Kramer, 2003; Chanpattana & Sackeim, 2010; Davarinejad et al., 2019; Ipekcioglu et al., 2018; Jia et al., 2019; Kim et al., 2018; Pawełczyk et al., 2014a, 2014b; Petrides et al., 2015; Tang & Ungvari, 2002, 2003; Üçok & Çakir, 2006; Xiang et al., 2015; Zhang et al., 2012).
The ages of the patients in the included publications ranged from 15 to 75 years. Depending on the country, where the study took place, there are patient cohorts from different populations within Asian and Middle Eastern (China, Hong Kong, India, Iran, South Korea, Thailand, Turkey), and Western (Germany, Italy, Poland, Netherlands, Turkey, United Kingdom, USA) countries. Excluding single cases in case reports, the sample size of studies varied considerably from 8 (Tang & Ungvari, 2001) to 6761 participants (Xiang et al., 2015) (see Table 1).
Treatment Plans
In 16 of the 21 studies, the patients received ECT three times a week in the acute phase (Chanpattana & Chakrabhand, 2001a; Chanpattana & Kramer, 2003; Chanpattana & Sackeim, 2010; Ipekcioglu et al., 2018; Jia et al., 2019; Kho et al., 2004; Kim et al., 2018; Masoudzadeh et al., 2007; Pawełczyk et al., 2014a, 2014b; Petrides et al., 2015; Tang & Ungvari, 2001, 2002, 2003; Üçok & Çakir, 2006; Zhang et al., 2012). In two other studies, ECT was administered twice weekly (Kho et al., 2004; Usta Saglam et al., 2020), in one study every other day up to ten ECTs (Li et al., 2016) and in one study according to a protocol with increasing time periods between the ECTs (Bansod et al., 2018). Some studies also decreased the weekly frequency of ECT according to the patient’s condition treatment response (Chanpattana & Kramer, 2003; Kim et al., 2017; Kim et al., 2018). In one study (Davarinejad et al., 2019), 8-day daily treatment with ECT was investigated. The overall number of acute ECT sessions varied among the studies between 8 to 20 sessions. Some studies included a plan for the patients to receive continuation (C-ECT - last up to 6 months; (Chanpattana et al., 1999c) or maintenance ECT (M-ECT - begins after ECT; (Chanpattana, 2000)) after the acute course (Chanpattana & Andrade, 2006; Chanpattana & Chakrabhand, 2001a, 2001b). A treatment benefit from one of the respective treatment plans could not be found in the comparison of the studies.
In 14 of the 21 studies, the ECT protocols specified bilateral electrode placement (Chanpattana & Chakrabhand, 2001a; Chanpattana & Kramer, 2003; Chanpattana & Sackeim, 2010; Ipekcioglu et al., 2018; Kim et al., 2018; Li et al., 2016; Pawełczyk et al., 2014a, 2014b; Petrides et al., 2015; Tang & Ungvari, 2001, 2002, 2003; Üçok & Çakir, 2006; Usta Saglam et al., 2020; Zhang et al., 2012), while four specified unilateral use of the electrodes (Davarinejad et al., 2019; Jia et al., 2019; Kho et al., 2004; Masoudzadeh et al., 2007). The focus of Bansod et al. (2018) was to compare three groups with different electrode placement options (i.e. unilateral, bitemporal and bifrontal) to each other and didn’t find any significant difference between the groups. One study did not specify the placement of the electrodes (Xiang et al., 2015). The definition of an adequate minimum generalized motorized seizure duration ranged between 20 (Ipekcioglu et al., 2018; Jia et al., 2019; Kho et al., 2004; Usta Saglam et al., 2020; Zhang et al., 2012) and 30 s (Chanpattana & Sackeim, 2010; Davarinejad et al., 2019; Pawełczyk et al., 2014a, 2014b), but was not mentioned in all papers, so that no concrete preference for treatment can be given. According to the anesthesia protocols (17/21) in six out of ten thiopental was used (Chanpattana & Chakrabhand, 2001a; Chanpattana & Sackeim, 2010; Kho et al., 2004; Kim et al., 2018; Tang & Ungvari, 2002, 2003), in nine propofol (Bansod et al., 2018; Davarinejad et al., 2019; Ipekcioglu et al., 2018; Jia et al., 2019; Li et al., 2016; Pawełczyk et al., 2014a, 2014b; Üçok & Çakir, 2006; Usta Saglam et al., 2020) and in two methohexital (Petrides et al., 2015; Zhang et al., 2012) was used as an anesthetic agent. In two of the studies (Pawełczyk et al., 2014a, 2014b), etomidate was used instead of propofol in patients who experienced short episodes of seizures despite the high charge used. A treatment benefit on negative symptoms by using a specific anesthetic could not be found in the comparison of the studies.
Except for one study (Tang & Ungvari, 2001), other intervention studies (Bansod et al., 2018; Chanpattana & Chakrabhand, 2001a; Chanpattana & Kramer, 2003; Chanpattana & Sackeim, 2010; Ipekcioglu et al., 2018; Jia et al., 2019; Kho et al., 2004; Kim et al., 2018; Li et al., 2016; Pawełczyk et al., 2014a, 2014b; Tang & Ungvari, 2002, 2003; Üçok et al., 2006; Usta Saglam et al., 2020; Xiang et al., 2015; Zhang et al., 2012) mentioned ECT treatment in combination with concurrent antipsychotic medication treatment. Antipsychotics given were not always explicitly listed and comprised amisulpride (Pawełczyk et al., 2014a, 2014b), aripiprazole (Davarinejad et al., 2019; Jia et al., 2019; Li et al., 2016), clozapine (Ahmed et al., 2017; Bansod et al., 2018; Davarinejad et al., 2019; Kho et al., 2004; Kim et al., 2018; Masoudzadeh et al., 2007; Pawełczyk et al., 2014a, 2014b; Petrides et al., 2015; Tang & Ungvari, 2002; Usta Saglam et al., 2020; Zhang et al., 2012), diazepam (Chanpattana & Sackeim, 2010), flupenthixol (Ahmed et al., 2017; Chanpattana & Chakrabhand, 2001a; Chanpattana & Kramer, 2003; Chanpattana & Sackeim, 2010), haloperidol (Jia et al., 2019; Üçok & Çakir, 2006; Zhang et al., 2012), loxapine (Ahmed et al., 2017), olanzapine (Ahmed et al., 2017; Jia et al., 2019; Li et al., 2016; Pawełczyk et al., 2014a, 2014b; Tang & Ungvari, 2002; Üçok et al., 2006; Zhang et al., 2012), quetiapine (Li et al., 2016; Pawełczyk et al., 2014a, 2014b; Zhang et al., 2012), risperidone (Ahmed et al., 2017; Jia et al., 2019; Li et al., 2016; Tang & Ungvari, 2002; Üçok & Çakir, 2006; Zhang et al., 2012), sulpiride (Ahmed et al., 2017; Zhang et al., 2012), ziprasidone (Jia et al., 2019; Pawełczyk et al., 2014a, 2014b) and zuclopentixol (Üçok & Çakir, 2006). One study (Jia et al., 2019) compared ECT to a treatment other than medication, which was electroacupuncture. Among other things evidence suggests ECT combined with antipsychotic drugs results in greater improvement of negative symptoms than with antipsychotic drugs or ECT alone (Ahmed et al., 2017; Chanpattana, 2007; Kho et al., 2004; Masoudzadeh et al., 2007; Pawełczyk et al., 2014a, 2014b; Tharyan & Adams, 2005).
Clinical Interview Types
The studies assessed changes in negative and positive symptoms using various clinical interview types and symptom scales, including negative symptom scores. The interviews were always done at a baseline before ECT and after that at varying time points.
The used interviews were the BPRS in 20/35 (Ahmed et al., 2017; Chanpattana, 2007; Chanpattana & Andrade, 2006; Chanpattana & Chakrabhand, 2001a; Chanpattana & Kramer, 2003; Chanpattana & Sackeim, 2010; Davalos et al., 2005; Davarinejad et al., 2019; Ipekcioglu et al., 2018; Lehnhardt et al., 2012; Martinotti et al., 2011; Overall & DR, 1962; Petrides et al., 2015; Sinclair et al., 2019; Tang & Ungvari, 2001, 2002, 2003; Tharyan & Adams, 2005; Üçok & Çakir, 2006; Zheng et al., 2016) and the PANSS in 18/35 papers (Ahmed et al., 2017; Bansod et al., 2018; Davarinejad et al., 2019; Kay et al., 1987; Kho et al., 2004; Kim et al., 2018; Lehnhardt et al., 2012; Li et al., 2016; Martinotti et al., 2011; Masoudzadeh et al., 2007; Moeller et al., 2017; Park & Lee, 2014; Pawełczyk et al., 2014a, 2014b; Seethalakshmi et al., 2006; Sinclair et al., 2019; Usta Saglam et al., 2020; Zhang et al., 2012; Zheng et al., 2019). The SANS (Andreasen, 1984; Petrides et al., 2015; Sinclair et al., 2019; Üçok & Çakir, 2006), which focuses entirely on negative symptoms, was used in 5/35 of the studies (Ipekcioglu et al., 2018; Jia et al., 2019; Tang & Ungvari, 2001, 2002, 2003). The Nurses’ observation scale for inpatient evaluation (NOSIE-30) (Honigfeld et al., 1966) is the only one administered by nurses (Tang & Ungvari, 2002, 2003).
Outcomes Concerning Negative Symptoms
The included studies reported divergent outcomes concerning the effects of ECT on negative symptoms. The available PANSS, BPRS and SANS-scores of individual studies can be found in Table 1. Eight of the studies showed no significant effect of ECT on negative symptoms for patients with schizophrenia (Chanpattana & Chakrabhand, 2001a; Chanpattana & Sackeim, 2010; Masoudzadeh et al., 2007; Petrides et al., 2015; Tang & Ungvari, 2002; Üçok & Çakir, 2006; Xiang et al., 2015; Zhang et al., 2012). However, two of those studies observed a worsening of negative symptoms in the group of patients that were non-responders to ECT during the trial (Chanpattana & Chakrabhand, 2001a; Chanpattana & Sackeim, 2010). Thirteen studies reported a significant reduction of negative symptoms in the patients treated with ECT (Bansod et al., 2018; Chanpattana & Kramer, 2003; Davarinejad et al., 2019; Ipekcioglu et al., 2018; Jia et al., 2019; Kho et al., 2004; Kim et al., 2018; Li et al., 2016; Pawełczyk et al., 2014a, 2014b; Tang & Ungvari, 2001, 2003; Usta Saglam et al., 2020). During the maintenance ECT phase, Tang and Ungvari (2003) found an increase of negative symptoms but overall a significant reduction of negative symptoms after the complete ECT treatment. The four case reports also recorded a reduction of negative symptoms after ECT treatment (Martinotti et al., 2011; Moeller et al., 2017; Park & Lee, 2014; Seethalakshmi et al., 2006). The eight reviews and the two meta-analyses came to mixed conclusions about the effect of ECT on negative symptoms. Citing insufficient data, Hasan et al. (2015b), Remington et al. (2016), Ahmed et al. (2017) and Dokucu (2015) did not draw final conclusions on the effect of ECT on negative symptoms. It was concluded by Tharyan and Adams (2005) that a small amount of literature shows a significant reduction of negative symptoms by ECT and a better effect of the combination of ECT and antipsychotics compared to monotherapy (Tharyan & Adams, 2005), according to Ahmed et al. (2017) especially in combination with clozapine. On the other hand, Chanpattana and Andrade (2006), Lehnhardt et al. (2012), Chanpattana (2007), Sinclair et al. (2019), and Zheng et al. (2016) did not see a clear improvement of negative symptoms following ECT, but still recommend it as a treatment option for schizophrenic patients who have exhausted pharmacological treatment (Chanpattana, 2007).
Three publications mentioned an observed effect on the different subscales of negative symptoms (Chanpattana & Chakrabhand, 2001a; Chanpattana & Sackeim, 2010; Ipekcioglu et al., 2018). Chanpattana’s studies focusing on the prediction of response to ECT assessed by the BPRS observed no effect or worsening of the specific negative symptoms on motor retardation, blunted affect and disorientation following ECT (Chanpattana & Chakrabhand, 2001a; Chanpattana & Sackeim, 2010). The same studies also found that high baseline scores for negative symptoms were predictive of non-response to ECT. Among the negative symptoms, the association with non-response was most marked for disorientation, emotional withdrawal, and modest for blunted affect (Chanpattana & Sackeim, 2010). Using the SANS, Ipekcioglu et al. (2018) reported the most rapid response and a significant reduction, particularly for the item affective flattening or blunted affect across all SANS sub-scores during ECT treatment. Compared with baseline scores, all SANS scores were lower after subsequent ECT treatments, with a particularly marked decrease occurring between sessions two and four. For the negative symptom domains alogia, avolition-apathy, and anhedonia-asociality, significant reductions were observed at every session compared to baseline. For lack of attention, a significant reduction compared to baseline could be observed from session 4 onwards.
Discussion
Principal Findings
Eighteen of the 35 (52%) included papers reported a positive effect of ECT on negative symptoms. However, the included studies have not substantially reduced the uncertainty surrounding the efficacy of ECT on negative symptoms. Studies were, for example, affected by small sample sizes, lack of control groups and offered sham treatments that affected their validity and reliability.
Effect on Negative Symptoms
Overall, the studies suggest that combination therapy of ECT and antipsychotic medication is associated with better outcomes than monotherapy (Ahmed et al., 2017; Chanpattana et al., 1999b; Kho et al., 2004; Masoudzadeh et al., 2007; Pawełczyk et al., 2014a, 2014b; Tharyan & Adams, 2005). Generally, more studies from the period 2000–2021 demonstrate favourable results on negative symptoms compared to studies demonstrating a worsening or no change in negative symptoms. However, many studies showed small effect sizes, 14/21 of the studies had a sample size smaller than 100 included patients, and the effect on negative symptoms was almost always more modest than that on positive or depressive symptoms. The case reports’ findings support the idea of ECT reducing negative symptoms in patients with schizophrenia (Martinotti et al., 2011; Moeller et al., 2017; Park & Lee, 2014; Seethalakshmi et al., 2006).
As we were especially interested in the effect of ECT on negative symptoms of schizophrenia, it is essential to examine the nature of the individual samples. In the studies reviewed, only two of them focused explicitly on negative symptoms (Pawełczyk et al., 2014a, 2014b). The included reviews (Chanpattana, 2007; Chanpattana & Andrade, 2006; Dokucu, 2015; Hasan et al., 2015b; Lehnhardt et al., 2012; Remington et al., 2016; Sinclair et al., 2019; Tharyan & Adams, 2005) provided an overview of ECT for schizophrenia, especially for the time period before 2000, which was helpful to see the development of studies on this topic. Nonetheless, our literature review focuses on ECT for negative symptoms and there is not much research published on the topic before the respective period of time. As previously discussed, 19/21 of the original studies focused on patients with chronic schizophrenia and treatment resistance (Ahmed et al., 2017; Bansod et al., 2018; Chanpattana & Andrade, 2006; Chanpattana & Chakrabhand, 2001a; Chanpattana & Kramer, 2003; Chanpattana & Sackeim, 2010; Davarinejad et al., 2019; Jia et al., 2019; Kho et al., 2004; Kim et al., 2018; Li et al., 2016; Masoudzadeh et al., 2007; Pawełczyk et al., 2014a, 2014b; Petrides et al., 2015; Tang & Ungvari, 2001, 2002, 2003; Usta Saglam et al., 2020). Since this group of patients is challenging to treat and often has negative and cognitive symptoms, examining the different aspects of symptomology in further detail may be helpful. Along these lines, Pawełczyk et al. (2014a) and Pawełczyk et al. (2014b) specifically examined treatment-resistant schizophrenia patients with dominant negative symptoms. The study found that the combination therapy of ECT and antipsychotics was most effective, especially in the early stages of treatment-resistant schizophrenia development, also with predominantly negative symptoms (Pawełczyk et al., 2014a), which should be noted for future treatment regimes. The current episode’s duration was found to be the only significant predictor of treatment response (Pawełczyk et al., 2014a, 2014b). Results corroborated this finding by Chanpattana’s studies involving treatment-resistant patients with predominantly positive symptoms (Chanpattana & Chakrabhand, 2001a; Chanpattana & Sackeim, 2010). It still could be seen that the baseline severity of negative symptoms has an impact on the outcome (Kendell, 1981; Tang & Ungvari, 2002). Nonetheless the authors recommended ECT for schizophrenic patients who have exhausted pharmacological treatment (Chanpattana, 2007).
As discussed, standardization of clinical interview types across studies would enable a more meaningful comparison of results. Along these lines of investigation, Ipekcioglu et al. (2018) found that especially emotional withdrawal and blunted affect were reduced by ECT treatment, along with improvements on other negative symptoms. This highlights the importance of scrutinizing changes within the subscales of negative symptoms and considering a differentiation between primary and secondary negative symptoms (Kirkpatrick et al., 2006; Remington et al., 2016).
It is also helpful to have a closer look at the results concerning negative symptoms and their development throughout ECT treatment (Chanpattana & Kramer, 2003). Chanpattana and Kramer (2003) showed the susceptibility of the results by various factors of the study design. However, it should be noted that the inclusion criteria for this study included a history of ECT response. This raises the question of whether early treatment is integral for better outcomes after all. It shows the importance of looking at the samples in detail. The only study using an intensive 8-day daily course of ECT, Davarinejad et al. (2019) found that TRS patients had reduced negative symptoms in the immediate period after ECT. However, these increased again between 4 and 12 weeks following the intervention. A worsening of negative symptoms in some of the patients in Chanpattana and Chakrabhand (2001a) was discussed by the authors due to the described cognitive adverse effects of ECT and should be evaluated considering secondary negative symptoms in future research. Considering predictors of response to treatment, Chanpattana and Chakrabhand (2001a) and Chanpattana and Sackeim (2010) for example, also showed that non-responders to ECT had a higher score of negative symptoms at baseline. Interestingly, the ECT treatment had no significant effect on negative symptoms across the total sample of the studies, but resulted in the improvement of negative symptoms in the group of treatment responders after all (Chanpattana & Chakrabhand, 2001a; Chanpattana & Sackeim, 2010). This suggests that the study sample’s baseline symptom profiles contribute to the likelihood of responding to ECT and the study outcome.
In agreement with Krueger and Sackheim (1995), giving patients with chronic schizophrenia a course of ECT as a treatment option should be considered, if pharmacological treatment options have been exhausted. Another advantage of the treatment with ECT are minor side effects; mainly those were transient cognitive impairments (Braga & Petrides, 2005; Sanghani et al., 2018). Some papers have suggested that severe negative symptoms are a predictor of adverse outcomes in general for ECT if applied to patients with schizophrenia (Agarwal et al., 1992; Chanpattana & Andrade, 2006; Chanpattana & Chakrabhand, 2001a; Freeman, 1995; Payne & Prudic, 2009; Xiang et al., 2015). This may lead to patients with negative symptoms receiving ECT less frequently. The study results of Xiang et al. (2015) indicate that patients with negative symptoms generally did not receive ECT, as negative symptoms were viewed as a predictor of inadequate ECT response. This study is also somewhat unique among the included studies as it attempts to provide an overview of ECT use in Asia. Most of the published research on ECT has been conducted in North American or European settings, and little is known about ECT in other regions. In Asia, despite schizophrenia being the most common indication for ECT, there is a substantial lack of studies about the use of ECT for schizophrenia (Xiang et al., 2015), especially on an international level. Also, when comparing the outcomes of international studies on this topic, the issues of comparing the treatment plans and study designs regionally and generally should be strongly considered.
Difficulties of Comparisons and Evaluating the Effectiveness of ECT
As discussed in Chanpattana and Andrade (2006), clinical research on ECT for schizophrenia has historically been severely limited by a lack of controlled, blinded and randomized trials (Andrade, 2016; Braga & Petrides, 2005; Chanpattana & Andrade, 2006; Sackeim, 2003; Salzman, 1980). Related studies often suffer from an incomplete description of samples, inappropriate ECT stimulus characteristics, lack of use of standard rating instruments, or small sample sizes. These issues are still commonly found even in recent studies. A closer look at the studies reveals some possible explanations for differing results. It highlights some important limitations of the studies as well as the difficulties of comparison and evaluating effectiveness.
Sample
The validity of many of the included studies suffered from small sample sizes (Button et al., 2013) and high dropout rates (e.g. Ipekcioglu et al., 2018; Masoudzadeh et al., 2007; Pawełczyk et al., 2014a, 2014b; Tang & Ungvari, 2002, 2003). Also, the baseline demographics and inclusion criteria varied between the studies. This could have had an impact on the treatment effect concerning the extent of negative symptoms at the end. Except two of the studies only including male patients (Ipekcioglu et al., 2018; Xiang et al., 2015) and two of them not giving detailed information on the gender of the participants (Chanpattana & Kramer, 2003; Kim et al., 2018), the rest of the studies included patients of both gender (Davarinejad et al., 2019). All in all, there is still a lack of gender and age comparisons in this research area, which needs to be kept in mind for future studies.
Considering the implications of Pawełczyk et al. (2014b) that ECT treatment had the most effect in the stage of the early development of TRS, it is surprising the only two studies having a look at the effect of ECT on patients with a first psychotic episode did not find a significant effect on negative symptoms (Üçok & Çakir, 2006; Zhang et al., 2012). However, it should also be kept in mind that negative symptoms are often present to an even lesser extent at the onset of the disease and a significant reduction is therefore more difficult to achieve in studies. When comparing the outcomes of the included studies, it is important to consider each of the different patient samples’ characteristics. Since the inclusion criteria and baseline characteristics of the patient groups were heterogeneous between some of the identified studies, it is difficult to draw meaningful comparisons between the studies (Krueger & Sackheim, 1995). Additional biomarkers to determine new endophenotypes and schizophrenic subtypes could improve precision in the measurement and treatment of schizophrenia disorders compared to existing subtypes (e.g. paranoid-delusional, hebephrenic and catatonic) (Keeley & Gaebel, 2018; Li et al., 2016; Weickert et al., 2013).
Treatment Plans and Use of Antipsychotics
It is difficult to draw meaningful comparisons between the effects of the investigated treatment and the study outcomes, with significant baseline variables being unspecified or sufficiently controlled. For example, age, duration of illness, number of previous psychotic episodes, previous hospitalizations, current hospital stay, the proportion of patients receiving medication, or neurocognitive status have not been recorded or controlled in many of the included studies. Also, information on electrode placement or anesthetic agents as part of the treatment was often missing. However, considering the studies that reported electrode placement, no significant advantage of uni- or bilateral electrode placement could be seen so far. By a comparison of groups with different electrode placements, Bansod et al. (2018) could even explicitly demonstrate that there was no benefit on symptoms – especially on negative symptoms – from any of the placement options. A treatment benefit on negative symptoms by using a specific anesthetic could also not be found comparing the studies to each other. However, none of the included studies investigated explicitly a possible interaction of anesthesia and negative symptoms. Finally researchers should focus on developing standardized treatment plans to allow for a more meaningful comparison of treatment outcomes (Sanghani et al., 2018). Treatment protocols varied significantly between the different studies, for example, concerning the number of ECT sessions, seizure threshold, and use of maintenance therapy. However, in a synopsis of the treatment plans of the individual studies, including the spacing and frequency of ECT sessions no significant advantage on the symptoms of any of the treatment protocols could be found so far. It could be shown by Chanpattana et al. (1999a) that thrice weekly ECT speeds response faster than does twice weekly ECT in the treatment of schizophrenic patients (Chanpattana, 2007; Chanpattana et al., 1999b). However, it was not considered, whether this also led to stronger neurocognitive side effects, which can be assumed. In major depression, twice-weekly ECT produces less severe short-term cognitive side effects but results in slower clinical improvement than thrice-weekly ECT (Chanpattana, 2007; Lerer et al., 1995; Shapira et al., 1998). The studies also varied greatly in their duration of follow up observations (Kim et al., 2018). An extended observation period is often missing to explore the need for maintenance or continuation treatment (Iancu et al., 2015; Kim et al., 2018) and to study the duration of remission of negative symptoms (Masoudzadeh et al., 2007; Tang & Ungvari, 2002, 2003). After an increase of negative symptoms following the immediate intervention period, Davarinejad et al. (2019) suggested that booster sessions of ECT might be beneficial (Davarinejad et al., 2019), which needs further investigation as a treatment option in the future. It has been suggested by Chanpattana (2007), that the use of continuation ECT combined with antipsychotic medication for patients who respond to a course of acute ECT is superior to continuation treatment with antipsychotics alone or continuation ECT alone in preventing relapses (Chanpattana, 2007; Chanpattana et al., 1999c) and that schizophrenic patients with more good prognosis factors require less frequent C-ECT (Chanpattana, 2007; Chanpattana & Chakrabhand, 2001b). There is also evidence that acute and maintenance ECT combined with antipsychotic medication is effective in improving negative symptoms as well as social functioning and quality of life in patients with TRS and history of responsiveness to ECT (Chanpattana, 2007; Chanpattana & Kramer, 2003). Here, the improvement in social functioning and quality of life could go hand in hand with the improvement in negative symptoms, although this has not yet been conclusively clarified. Nevertheless, as Chanpattana (2007) already mentioned in one of his reviews, it cannot be denied that issues such as electrode placement, stimulus dosing, treatment frequency, and cognitive side effects deserve better investigation in the context of schizophrenia (Chanpattana, 2007).
In all studies except one (Masoudzadeh et al., 2007), ECT was administered in combination with pharmacological treatment and lacked a medication-free comparison group. Control groups receiving no medication would be required to study the effect of ECT as a single treatment. Medication regimes included mono- or polypharmaceutical treatment with various first- or second-generation antipsychotics, often without standardized doses, plasma levels, or treatment length (Jia et al., 2019; Kim et al., 2018; Pawełczyk et al., 2014a, 2014b; Tang & Ungvari, 2002, 2003; Xiang et al., 2015). Conducting a study with a similar controlled design as (Masoudzadeh et al., 2007) and with a larger sample size should be considered. As mentioned, only one study compared ECT to antipsychotics and another treatment form (i.e., electroacupuncture) (Jia et al., 2019). Included studies on the topic suggest a superiority of therapies with a combination of ECT and antipsychotics over a monotherapy of ECT or antipsychotics alone. This is likely due to synergistic effects of both therapy forms (Breier et al., 1987; Chanpattana et al., 1999c; Garg et al., 2012; Goldberg, 1985; Krueger & Sackheim, 1995; Tharyan & Adams, 2005). For example, a disruption of the blood-brain-barrier by the ECT treatment might result in stronger effects by the antipsychotics directly in the brain (Jahangard et al., 2012).
Methods
An important limitation that should be addressed in future studies was the use of uncontrolled and non-randomized methods without blinding (Braga & Petrides, 2005; Ipekcioglu et al., 2018; Tang & Ungvari, 2001, 2003). Only eleven of the studies (Bansod et al., 2018; Jia et al., 2019; Kim et al., 2018; Li et al., 2016; Masoudzadeh et al., 2007; Petrides et al., 2015; Tang & Ungvari, 2001, 2002; Üçok & Çakir, 2006; Usta Saglam et al., 2020; Zhang et al., 2012) had control groups, and only one study used a sham treatment as a placebo group (Masoudzadeh et al., 2007). In two studies, the control groups consisted of patients who had refused ECT (Tang & Ungvari, 2002, 2003). In both of these studies, the authors acknowledged that refusing ECT may, for example, be associated with a fear of complications, distrust in the treatment, or paranoid symptoms at baseline and thus may have influenced the results (Tang & Ungvari, 2002). Additionally, only two studies mentioned a blinded assessor (Davarinejad et al., 2019; Petrides et al., 2015) and only one study was double-blinded (Masoudzadeh et al., 2007).
It is difficult to compare the interview types generally since they use different subscales for negative symptoms and are based on different definitions of negative symptoms (Andreasen, 1984; Foussias et al., 2014; Foussias et al., 2015; Kirkpatrick et al., 2006; Marder & Galderisi, 2017; Remington et al., 2016). As mentioned, only three publications observe an effect on the different subscales of negative symptoms using the BPRS (Chanpattana & Chakrabhand, 2001a; Overall & DR, 1962) or the SANS (Andreasen, 1984; Ipekcioglu et al., 2018). However, the BPRS score includes a negative symptom score of emotional withdrawal, motor retardation, blunted affect and disorientation (Chanpattana & Kramer, 2003; Davarinejad et al., 2019). The PANSS (Kay et al., 1987) is somewhat lengthier, aiming to quantify the main negative symptoms: blunted affect, emotional withdrawal, poor rapport, passive/apathetic, difficulty in abstract thinking, lack of spontaneity and flow of conversation, and stereotyped thinking (Davarinejad et al., 2019). The SANS (Andreasen, 1984) is the only assessment focusing entirely on negative symptoms and consists of five subscales for negative symptoms: emotional flattening or blunting, anhedonia, alogia, avolition and lack of attention (Andreasen, 1984). The NOSIE-30 (Honigfeld et al., 1966) is administered by nurses focusing on behaviour at the ward and therefore focuses on a different perspective of negative symptoms than other interviews. According to Chanpattana (2007), the BPRS is not suitable for recording an improvement in negative symptoms through ECT. Since treatment response usually occurs as a continuum and there is no line of demarcation (Barnes et al., 2003; Chanpattana, 2007; Kane, 1996), defining response criteria by setting a minimum percentage of decrement in the BPRS should not be used (Chanpattana, 2007; Overall & Gorham, 1962). This type of response criteria will create a highly differential effect. Patients with a wide range of BPRS scores would be included in the same group that subsequently had impact on the results of continuation/ maintenance studies (Chanpattana, 2007). Therefore, by choosing a suboptimal choice of the rating scale it could explain that the BPRS did not show any improvements in negative symptoms in the studies conducted by Chanpattana (Chanpattana & Chakrabhand, 2001a; Chanpattana & Sackeim, 2010). Also missing effects on the specific negative symptoms could especially be seen on motor retardation, disorientation, and blunted affect, whereby in the case of disorientation and motor retardation a clear differentiation from the cognitive side effects of ECT would be necessary. The lack of effect on blunted affect also contrasts with the findings of Ipekcioglu et al. (2018), who showed most rapid response and a significant reduction, particularly for the item affective flattening or blunted affect across all SANS sub-scores during ECT treatment. Instead of a broad assessment tool as the BPRS, the SANS could be used to investigate negative symptoms explicitly for the future.
Implications and Recommendations for Future Research and Practice
More studies on this topic and related symptoms are urgently needed to reduce the uncertainty surrounding the efficacy of ECT on negative symptoms. Unfortunately, the included literature reviews of Dokucu (2015), Hasan et al. (2015b), Lehnhardt et al. (2012) and Remington et al. (2016) do not provide detailed information on the treatment plans of their included studies, so that no comparison could be made with our review in this respect. Preliminary searches of databases used in this study for ECT and schizophrenia with manifestations of either positive, negative, depressive, manic, psychomotor, or cognitive symptoms reveals that some symptoms receive little research attention, especially in comparison to other co-occurring symptoms (see Fig. 2).
The following recommendations regarding research on ECT treatment for patients with schizophrenia should be implemented according to the general guidelines established by Chanpattana (2007) and supplemented with aspects regarding negative symptoms. Negative symptoms showed no clear improvement in the studies and reviews of Chanpattana et al. (Chanpattana, 2007). However, it must be noted that in the large number of papers and reviews published by the authors, their own study results were often cited repeatedly, so that the significance regarding the effects of ECT on negative symptoms can be considered limited due to a bias. Nevertheless, many of the general statements regarding the recommendations for future research on ECT treatment in schizophrenic patients should be highlighted since unfortunately some recommendations remain similar, as there has been little movement in this area.
The problems regarding methodology of prior studies should be corrected as much as possible and ECT treatment techniques should follow the respective current standard guidelines. Standardization of treatment plans and clinical interview types with the same subscales of negative symptoms across ECT studies would enable a more meaningful comparison of results (Foussias et al., 2014). Comparing study designs and outcomes regionally and on an international level in the form of multicentered studies should be considered. Well-designed, randomized controlled trials (RCT) including larger sample sizes and sham treatments should be conducted to increase validity and reliability and to compare the efficacy of short term and/or long-term ECT treatments with antipsychotics. As Sinclair et al. (2019) also concludes in his systematic review more good-quality evidence is needed before firm conclusions can be made. Blind assessments should be used, and inter-rater reliability tests should be done regularly. Response criteria must be clearly defined with appropriate reasons and should not be limited by the number of ECT treatments. To draw meaningful comparisons between the studies, similar inclusion criteria and baseline characteristics of the patient group are necessary. In addition, there is still a lack of gender and age comparisons in the research area. Since the treatment for the group of patients with chronic schizophrenia and treatment resistance is challenging, focusing on the different aspects of symptomology in further detail might be helpful. Also relapse criteria must be clearly defined (Chanpattana, 2007).
The studies suggest a benefit of combination therapy with ECT and antipsychotics over monotherapy; however, this should be tested in a controlled study. Otherwise ECT treatment should always be prescribed in combination with antipsychotics, also with a treatment focus is on negative symptoms, as studies have generally shown a clear advantage of combination treatment over monotherapy. Additionally, more studies concerning negative symptoms should compare ECT to or in combination with other non-medication treatment forms.
In addition, ECT treatment should focus strongly on the individual subgroups of negative symptoms. In particular, in the studies with attention to the subgroups, there was an effect on blunted affect or affective flattening (Ipekcioglu et al., 2018), which is a very limiting symptom for the individual. Among other things, this correlates with impaired emotion recognition (Gur et al., 2006) and thus leads to difficulties in social interactions and exclusion from society (Marder & Galderisi, 2017). Thus, even an improvement of this symptom component would be a success for each individually affected patient. Since according to Chanpattana (2007), the BPRS is not suitable for recording an improvement in negative symptoms through ECT (Chanpattana, 2007), instead the SANS should be used to investigate negative symptoms. Before an ECT indication is made, it should also be clearly worked out which negative subdomains are present in the patient. In addition, a distinction should be made between primary and secondary negative symptoms as well as depressive symptoms, and the severity of baseline negative symptoms should also be taken into account (Chanpattana, 2007). There is also a need for additional biomarkers to determine new endophenotypes and schizophrenic subtypes. This could improve precision in the measurement and treatment of schizophrenia disorders compared to existing subtypes (e .g. paranoid-delusional, hebephrenic and catatonic) based on clinical observations (Keeley & Gaebel, 2018; Weickert et al., 2013).
As far as we can now take along for future practice of ECT for schizophrenic patients with negative symptoms a combination with antipsychotics is highly recommended. The general recommendation regarding the interval between ECT treatments is currently three times a week. The choice of anaesthetic used and electrode placement does not seem to play a role. In principle, ECT treatment seems to be beneficial when used earlier in the course of the disease, before pronounced chronification and newer assessments such as the SANS should be used to assess progression.
Limitations
The review covered the last 20 years, and other relevant publications could have been published prior. However, preliminary searches for ten years prior to the year 2000 did not reveal any relevant studies published before that time (Ward et al., 2018). Also, included study results were limited to publications in the English and German languages. Findings should be interpreted cautiously based on the small number of included studies (35). In principle, a meta-analysis for a more detailed evaluation could be considered as a next step, although with the current data situation it is questionable how meaningful this would be at this stage. Due to the varying outcomes and treatment plans in the reviewed studies it is not possible at this stage to provide a definitive and universal treatment plan for negative symptoms in schizophrenia. Rather, there can only be a renewed call to promote standardisation and comparability in this area of research and to apply what we know so far, since some actionable insights could still be drawn.
Conclusion
In agreement with Krueger and Sackheim (1995), regardless of chronicity, patients with schizophrenia who have exhausted pharmacological alternatives could benefit from a course of ECT. The review highlighted the methodological shortcomings of the included publications that affected their validity and reliability. The significant variation of study designs and missing standardized protocols made it extremely difficult to compare the different results of the reviewed studies. Implications for practice and future research were also discussed and recommendations were provided towards this end. For instance, study designs that focus explicitly on negative symptoms and assess patients over longer follow up periods than so far would be helpful (Hasan et al., 2015a; Ipekcioglu et al., 2018; Tang & Ungvari, 2002). As many studies did not consider gender differences in the effect of ECT on patients with schizophrenia, it should be assessed by future research as well. Future research is vital to maintain comparability of results in mind, include control groups, and possibly establish international multicentered studies to get a sufficient study population. As far as one can recommend at this point, the future practice of ECT for negative symptoms in schizophrenia should include a combination treatment with antipsychotics. The general recommendation regarding the interval between ECT treatments is currently three times a week, whereas the use of anaesthetics, electrode placement and seizure threshold does not seem to play a role. The use of the SANS for the assessment of negative symptoms should be considered for the future and in principle, one should not be too hesitant in recommending ECT treatment in the case of a possible benefit for the individual patient, since after an overview of the data, a risk and benefit assessment generally speaks in favor of the treatment option.
(schizophrenia[Title/Abstract] OR psychosis[Title/Abstract] OR psychoses[Title/Abstract] OR ‘psychotic disorder’[Title/Abstract] OR ‘schizophrenic disorder’[Title/Abstract] OR ‘schizophrenia spectrum disorders’[Title/Abstract]) AND (‘electroconvulsive therapy’[Title/Abstract] OR ‘ect’[Title/Abstract] OR ‘shock therapy’[Title/Abstract] OR ‘electroshock therapy’[Title/Abstract] OR ‘electroshock’[Title/Abstract]) AND (‘negative symptoms’[Title/Abstract])
(TI (ect or electro convulsive therapy or shock therapy) OR SU (ect or electro convulsive therapy or shock therapy) OR AB (ect or electro convulsive therapy or shock therapy)) AND (TI (schizophrenia or psychosis or psychoses or psychotic disorder or schizophrenic disorder or schizophrenic disorder or schizophrenic spectrum or schizophrenia spectrum disorder) OR SU (schizophrenia or psychosis or psychoses or psychotic disorder or schizophrenic disorder or schizophrenic disorder or schizophrenic spectrum or schizophrenia spectrum disorder) OR AB (schizophrenia or psychosis or psychoses or psychotic disorder or schizophrenic disorder or schizophrenic disorder or schizophrenic spectrum or schizophrenia spectrum disorder)) AND (TI (lack of pleasure or withdrawal symptoms or emotional withdrawal or motor retardation or blunted affect or disorientation or alogia or blunting or anhedonia or avolition or lack of attention or negative symptoms) OR SU (lack of pleasure or withdrawal symptoms or emotional withdrawal or motor retardation or blunted affect or disorientation or alogia or blunting or anhedonia or avolition or lack of attention or negative symptoms) OR AB (lack of pleasure or withdrawal symptoms or emotional withdrawal or motor retardation or blunted affect or disorientation or alogia or blunting or anhedonia or avolition or lack of attention or negative symptoms))
References
Agarwal, A., Andrade, C., & Reddy, M. V. (1992). The practice of ECT in India: Issues relating yo the administration of ECT. Indian Journal of Psychiatry, 34(4), 285–297.
Ahmed, S., Khan, A. M., Mekala, H. M., Venigalla, H., Ahmed, R., Etman, A., Esang, M., & Qureshi, M. (2017). Combined use of electroconvulsive therapy and antipsychotics (both clozapine and non-clozapine) in treatment resistant schizophrenia: A comparative meta-analysis. Heliyon, 3(11), e00429. https://doi.org/10.1016/j.heliyon.2017.e00429.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing.
Andrade, C. (2016). Anti-inflammatory strategies in the treatment of schizophrenia. Expert review of clinical pharmacology, 9(2), 161–163.
Andreasen, N. (1984). Scale for the assessment of negative symptoms (SANS) Iowa City, IA: Univ. Iowa: 1984b.
Austin, S. F., Mors, O., Secher, R. G., Hjorthøj, C. R., Albert, N., Bertelsen, M., Jensen, H., Jeppesen, P., Petersen, L., & Randers, L. (2013). Predictors of recovery in first episode psychosis: The OPUS cohort at 10 year follow-up. Schizophrenia Research, 150(1), 163–168. https://doi.org/10.1016/j.schres.2013.07.031.
Bansod, A., Sonavane, S. S., Shah, N. B., De Sousa, A. A., & Andrade, C. (2018). A randomized, nonblind, naturalistic comparison of efficacy and cognitive outcomes with right unilateral, bifrontal, and bitemporal electroconvulsive therapy in schizophrenia. The Journal of ECT, 34(1), 26–30. https://doi.org/10.1097/yct.0000000000000454.
Barnes, T., Buckley, P., & Schulz, S. C. (2003). Treatment-resistant Schizophrenia. In Schizophrenia (pp. 489–516). https://doi.org/10.1002/9780470987353.ch26
Braga, R. J., & Petrides, G. (2005). The combined use of electroconvulsive therapy and antipsychotics in patients with schizophrenia. The Journal of ECT, 21(2), 75–83. https://doi.org/10.1097/01.yct.0000165500.60784.05.
Breier, A., Wolkowitz, O. M., Doran, A. R., Roy, A., Boronow, J., Hommer, D. W., & Pickar, D. (1987). Neuroleptic responsivity of negative and positive symptoms in schizophrenia. The American Journal of Psychiatry, 144(12), 1549–1555. https://doi.org/10.1176/ajp.144.12.1549
Button, K. S., Ioannidis, J. P., Mokrysz, C., Nosek, B. A., Flint, J., Robinson, E. S., & Munafò, M. R. (2013). Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14(5), 365–376. https://doi.org/10.1038/nrn3475.
Campellone, T. R., Caponigro, J. M., & Kring, A. M. (2014). The power to resist: The relationship between power, stigma, and negative symptoms in schizophrenia. Psychiatry Research, 215(2), 280–285.
Canadian Agency for Drugs and Technologies in Health. (2014). Delivery of electroconvulsive therapy in non-hospital settings: A review of the safety and guidelines. CADTH Rapid Response Reports.
Cerletti, U., & Bini, L. (1938). Un nuevo metode di shockterapie" L'elettro-shock". Reale Accademia Medica (Communicazione alla seduta del 28 maggio 1938-XVI della Reale Accademia Medica di Roma.), Rome, 64, 136–138.
Chanpattana, W. (2000). Maintenance ECT in treatment-resistant schizophrenia. Journal of the Medical Association of Thailand= Chotmaihet thangphaet, 83(6), 657–662.
Chanpattana, W. (2007). Electroconvulsive therapy for Schizophrenia. Current Psychiatry Reviews, 3(1), 15–24.
Chanpattana, W., & Andrade, C. (2006). ECT for treatment-resistant schizophrenia: A response from the far east to the UK. NICE report. The journal of ECT, 22(1), 4–12. https://doi.org/10.1097/00124509-200603000-00002.
Chanpattana, W., Chakrabhand, M., Kitaroonchai, W., Choovanichvong, S., & Prasertsuk, Y. (1999a). Effects of twice-versus thrice-weekly electroconvulsive therapy in schizophrenia. Journal of the Medical Association of Thailand= Chotmaihet thangphaet, 82(5), 477–483.
Chanpattana, W., Chakrabhand, M., Kongsakon, R., Techakasem, P., & Buppanharun, W. (1999b). Short-term effect of combined ECT and neuroleptic therapy in treatment-resistant schizophrenia. The Journal of ECT, 15(2), 129–139. https://doi.org/10.1097/00124509-199906000-00004
Chanpattana, W., Chakrabhand, M., Sackeim, H. A., Kitaroonchai, W., Kongsakon, R., Techakasem, P., Buppanharun, W., Tuntirungsee, Y., & Kirdcharoen, N. (1999c). Continuation ECT in treatment-resistant schizophrenia: A controlled study. The Journal of ECT, 15(3), 178–192. https://doi.org/10.1097/00124509-199909000-00002
Chanpattana, W., & Chakrabhand, M. S. (2001a). Combined ECT and neuroleptic therapy in treatment-refractory schizophrenia: Prediction of outcome. Psychiatry Research, 105(1–2), 107–115. https://doi.org/10.1016/S0165-1781(01)00321-3.
Chanpattana, W., & Chakrabhand, M. S. (2001b). Factors influencing treatment frequency of continuation ECT in schizophrenia. The Journal of ECT, 17(3), 190–194. https://doi.org/10.1097/00124509-200109000-00008.
Chanpattana, W., & Kramer, B. A. (2003). Acute and maintenance ECT with flupenthixol in refractory schizophrenia: Sustained improvements in psychopathology, quality of life, and social outcomes. Schizophrenia Research, 63(1–2), 189–193. https://doi.org/10.1016/S0920-9964(02)00330-4.
Chanpattana, W., & Sackeim, H. A. (2010). Electroconvulsive therapy in treatment-resistant schizophrenia: Prediction of response and the nature of symptomatic improvement. The Journal of ECT, 26(4), 289–298. https://doi.org/10.1097/yct.0b013e3181cb5e0f.
Davalos, D., Grutzendler, J., Yang, G., Kim, J. V., Zuo, Y., Jung, S., Littman, D. R., Dustin, M. L., & Gan, W. B. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nature Neuroscience, 8(6), 752–758. https://doi.org/10.1038/nn1472.
Davarinejad, O., Hendesi, K., Shahi, H., Brand, S., & Khazaie, H. (2019). A pilot study on daily intensive ECT over 8 days improved positive and negative symptoms and general psychopathology of patients with treatment-resistant Schizophrenia up to 4 weeks after treatment. Neuropsychobiology, 77(2), 83–91. https://doi.org/10.1159/000494698.
De Berardis, D., Rapini, G., Olivieri, L., Di Nicola, D., Tomasetti, C., Valchera, A., Fornaro, M., Di Fabio, F., Perna, G., & Di Nicola, M. (2018). Safety of antipsychotics for the treatment of schizophrenia: A focus on the adverse effects of clozapine. Therapeutic advances in drug safety, 9(5), 237–256.
Dijkers, M. P. (2009). The value of “traditional” reviews in the era of systematic reviewing. American Journal of Physical Medicine & Rehabilitation, 88(5), 423–430. https://doi.org/10.1097/phm.0b013e31819c59c6.
Ding, Z., & White, P. F. (2002). Anesthesia for electroconvulsive therapy. Anesthesia & Analgesia, 94(5), 1351–1364. https://doi.org/10.1097/00000539-200205000-00057.
Dokucu, M. E. (2015). Neuromodulation treatments for Schizophrenia. Curr Treat Options Psychiatry, 2(3), 339–348. https://doi.org/10.1007/s40501-015-0055-4.
Fink, M. (1994). The mode of action of ECT. Psychopharmacology Bulletin, 30(3), 309–312.
Fink, M., & Sackeim, H. A. (1996). Convulsive therapy in schizophrenia? Schizophrenia Bulletin, 22(1), 27–39. https://doi.org/10.1093/schbul/22.1.27.
Foussias, G., Agid, O., Fervaha, G., & Remington, G. (2014). Negative symptoms of schizophrenia: Clinical features, relevance to real world functioning and specificity versus other CNS disorders. European Neuropsychopharmacology, 24(5), 693–709. https://doi.org/10.1016/j.euroneuro.2013.10.017.
Foussias, G., Siddiqui, I., Fervaha, G., Agid, O., & Remington, G. (2015). Dissecting negative symptoms in schizophrenia: Opportunities for translation into new treatments. Journal of Psychopharmacology, 29(2), 116–126. https://doi.org/10.1177/0269881114562092.
Freeman, C. (1995). The ECT handbook: The second report of the Royal College of Psychiatrists' special committee on ECT. Royal College of Psychiatrists London.
Garg, R., Chavan, B. S., & Arun, P. (2012). Short-term efficacy of electroconvulsive therapy in treatment-resistant schizophrenia. German Journal of Psychiatry, 15(2), 44–49.
Goldberg, S. C. (1985). Negative and deficit symptoms in schizophrenia do respond to neuroleptics. Schizophrenia Bulletin, 11(3), 453–456. https://doi.org/10.1093/schbul/11.3.453.
Gur, R. E., Kohler, C. G., Ragland, J. D., Siegel, S. J., Lesko, K., Bilker, W. B., & Gur, R. C. (2006). Flat affect in schizophrenia: Relation to emotion processing and neurocognitive measures. Schizophrenia Bulletin, 32(2), 279–287.
Hasan, A., Falkai, P., Wobrock, T., Lieberman, J., Glenthøj, B., Gattaz, W. F., Thibaut, F., Möller, H.-J., & Schizophrenia, W. T. F. O. T. G. F. (2015a). World Federation of Societies of biological psychiatry (WFSBP) guidelines for biological treatment of schizophrenia part 3: Update 2015 management of special circumstances: Depression, suicidality, substance use disorders and pregnancy and lactation. The World Journal of Biological Psychiatry, 16(3), 142–170. https://doi.org/10.3109/15622975.2015.1009163.
Hasan, A., Wobrock, T., Palm, U., Strube, W., Padberg, F., Falkai, P., Fallgatter, A., & Plewnia, C. (2015b). Hirnstimulationsverfahren zur Behandlung schizophrener Psychosen. Der Nervenarzt, 86(12), 1481–1491. https://doi.org/10.1007/s00115-015-4323-8.
Honigfeld, G., Gillis, R. D., & Klett, C. J. (1966). NOSIE-30: A treatment-sensitive ward behavior scale. Psychological Reports, 19(1), 180–182. https://doi.org/10.2466/pr0.1966.19.1.180.
Iancu, I., Pick, N., Seener-Lorsh, O., & Dannon, P. (2015). Patients with schizophrenia or schizoaffective disorder who receive multiple electroconvulsive therapy sessions: Characteristics, indications, and results. Neuropsychiatric Disease and Treatment, 11, 853. https://doi.org/10.2147/ndt.s78919.
Ipekcioglu, D., Yazar, M. S., Canbek, O., Yuksel, O., Meterelliyoz, K. S., & Ilnem, M. C. (2018). Electroconvulsive therapy combined with antipsychotic therapy in the treatment of acute schizophrenia inpatients: Symptom profile of the clinical response. Psychiatry and Clinical Psychopharmacology, 28(4), 363–370. https://doi.org/10.1080/24750573.2018.1446729.
Jahangard, L., Haghighi, M., Bigdelou, G., Bajoghli, H., & Brand, S. (2012). Comparing efficacy of ECT with and without concurrent sodium valproate therapy in manic patients. The Journal of ECT, 28(2), 118–123. https://doi.org/10.1097/yct.0b013e31824b64b5.
Jia, J., Shen, J., Liu, F.-H., Wong, H. K., Yang, X.-J., Wu, Q.-J., Zhang, H., Wang, H.-N., Tan, Q.-R., & Zhang, Z.-J. (2019). Effectiveness of electroacupuncture and electroconvulsive therapy as additional treatment in hospitalized patients with schizophrenia: A retrospective controlled study. Frontiers in Psychology, 10, 2306. https://doi.org/10.3389/fpsyg.2019.02306.
Kane, J. M. (1996). Treatment-resistant schizophrenic patients. The Journal of Clinical Psychiatry, 57, 35–40.
Kay, S. R., Fiszbein, A., & Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261–276. https://doi.org/10.1093/schbul/13.2.261.
Keeley, J., & Gaebel, W. (2018). Symptom rating scales for schizophrenia and other primary psychotic disorders in ICD-11. Epidemiology and Psychiatric Sciences, 27(3), 219–224. https://doi.org/10.1017/s2045796017000270.
Kendell, R. (1981). The present status of electroconvulsive therapy. The British Journal of Psychiatry, 139(4), 265–283. https://doi.org/10.1192/bjp.139.4.265.
Kho, K., Blansjaar, B., De Vries, S., Babuskova, D., Zwinderman, A., & Linszen, D. (2004). Electroconvulsive therapy for the treatment of clozapine nonresponders suffering from schizophrenia. European Archives of Psychiatry and Clinical Neuroscience, 254(6), 372–379. https://doi.org/10.1007/s00406-004-0517-y.
Kim, H. S., Kim, S. H., Lee, N. Y., Youn, T., Lee, J. H., Chung, S., Kim, Y. S., & Chung, I. W. (2017). Effectiveness of electroconvulsive therapy augmentation on clozapine-resistant schizophrenia. Psychiatry Investigation, 14(1), 58–62. https://doi.org/10.4306/pi.2017.14.1.58.
Kim, J. H., Youn, T., Choi, J. G., Jeong, S. H., Jung, H. Y., Kim, Y. S., & Chung, I. W. (2018). Combination of electroconvulsive therapy and clozapine in treatment-resistant schizophrenia. Psychiatry investigation, 15(8), 829. https://doi.org/10.30773/pi.2018.05.15.
Kirkpatrick, B., Buchanan, R. W., Ross, D. E., & Carpenter, W. T. (2001). A separate disease within the syndrome of schizophrenia. Archives of General Psychiatry, 58(2), 165–171. https://doi.org/10.1001/archpsyc.58.2.165.
Kirkpatrick, B., Fenton, W. S., Carpenter, W. T., & Marder, S. R. (2006). The NIMH-MATRICS consensus statement on negative symptoms. Schizophrenia Bulletin, 32(2), 214–219. https://doi.org/10.1093/schbul/sbj053.
Krueger, R., & Sackheim, H. (1995). Electroconvulsive therapy and schizophrenia. Schizophrenia.
Lehman, A. F., Lieberman, J. A., Dixon, L. B., McGlashan, T. H., Miller, A. L., Perkins, D. O., Kreyenbuhl, J., McIntyre, J. S., Charles, S. C., & Altshuler, K. (2004). Practice guideline for the treatment of partients with schizophrenia. American Journal of psychiatry, 161(2 SUPPL.), i-iv+ 1-56.
Lehnhardt, F., Konkol, C., & Kuhn, J. (2012). Use of ECT in drug-refractory schizophrenia--a survey of the current literature. Fortschritte der Neurologie-Psychiatrie, 80(9), 501–511. https://doi.org/10.1055/s-0032-1312853.
Lerer, B., Shapira, B., Calev, A., Tubi, N., Drexler, H., Kindler, S., Lidsky, D., & Schwartz, J. E. (1995). Antidepressant and cognitive effects of twice-versus three-times-weekly ECT. The American journal of psychiatry., 152, 564–570. https://doi.org/10.1176/ajp.152.4.564.
Li, J., Ye, F., Xiao, W., Tang, X., Sha, W., Zhang, X., & Wang, J. (2016). Increased serum brain-derived neurotrophic factor levels following electroconvulsive therapy or antipsychotic treatment in patients with schizophrenia. European Psychiatry, 36, 23–28.
Marder, S. R., & Galderisi, S. (2017). The current conceptualization of negative symptoms in schizophrenia. World Psychiatry, 16(1), 14–24. https://doi.org/10.1002/wps.20385.
Martinotti, G., Ricci, V., Di Nicola, M., Caltagirone, C., Bria, P., & Angelucci, F. (2011). Brain-derived neurotrophic factor and electroconvulsive therapy in a schizophrenic patient with treatment-resistant paranoid-hallucinatory symptoms. The Journal of ECT, 27(1), e44–e46. https://doi.org/10.1097/yct.0b013e318205e1c0.
Masoudzadeh, A., Khalilian, A., & Hosseini, S. H. (2007). Comparative study of clozapine, electroconvulsive therapy (ECT), and the combination of ECT with clozapine in treatment-resistant schizophrenic patients. Pakistan Journal of Biological Sciences, 10, 4287–4290. https://doi.org/10.3923/pjbs.2007.4287.4290
McDonagh, M., Peterson, K., Raina, P., Chang, S., & Shekelle, P. (2013). Avoiding bias in selecting studies. In Methods guide for effectiveness and comparative effectiveness reviews [Internet]. Agency for Healthcare Research and Quality (US).
Moeller, S., Kalkwarf, N., Lücke, C., Ortiz, D., Jahn, S., Först, C., Braun, N., Philipsen, A., & Müller, H. H. (2017). Achieving stable remission with maintenance electroconvulsive therapy in a patient with treatment-resistant schizophrenia: A case report. Medicine, 96(48), e8813. https://doi.org/10.1097/MD.0000000000008813
Overall, J., & Dr, G. (1962). Overall & Gorham, 1962 BPRS psychol rep-799-812. Psychological Reports, 10, 799–812.
Overall, J. E., & Gorham, D. R. (1962). The brief psychiatric rating scale. Psychological Reports, 10(3), 799–812. https://doi.org/10.2466/pr0.1962.10.3.799.
Park, S., & Lee, M.-K. (2014). Successful electroconvulsive therapy and improvement of negative symptoms in refractory schizophrenia with clozapine-induced seizures: A case report. Psychiatria Danubina, 26(4), 0–362.
Pawełczyk, T., Kołodziej-Kowalska, E., Pawełczyk, A., & Rabe-Jabłońska, J. (2014a). Augmentation of antipsychotics with electroconvulsive therapy in treatment-resistant schizophrenia patients with dominant negative symptoms: A pilot study of effectiveness. Neuropsychobiology, 70(3), 158–164. https://doi.org/10.1159/000366484.
Pawełczyk, T., Kołodziej-Kowalska, E., Pawełczyk, A., & Rabe-Jabłońska, J. (2014b). Effectiveness and clinical predictors of response to combined ECT and antipsychotic therapy in patients with treatment-resistant schizophrenia and dominant negative symptoms. Psychiatry Research, 220(1–2), 175–180. https://doi.org/10.1016/j.psychres.2014.07.071.
Payne, N. A., & Prudic, J. (2009). Electroconvulsive therapy part I: A perspective on the evolution and current practice of ECT. Journal of Psychiatric Practice, 15(5), 346–368. https://doi.org/10.1097/01.pra.0000361277.65468.ef.
Petrides, G., Malur, C., Braga, R. J., Bailine, S. H., Schooler, N. R., Malhotra, A. K., Kane, J. M., Sanghani, S., Goldberg, T. E., & John, M. (2015). Electroconvulsive therapy augmentation in clozapine-resistant schizophrenia: A prospective, randomized study. American Journal of Psychiatry, 172(1), 52–58. https://doi.org/10.1176/appi.ajp.2014.13060787.
Powell, B. J., Proctor, E. K., & Glass, J. E. (2014). A systematic review of strategies for implementing empirically supported mental health interventions. Research on Social Work Practice, 24(2), 192–212. https://doi.org/10.1177/1049731513505778.
Remington, G., Foussias, G., Fervaha, G., Agid, O., Takeuchi, H., Lee, J., & Hahn, M. (2016). Treating negative symptoms in schizophrenia: An update. Current treatment options in psychiatry, 3(2), 133–150. https://doi.org/10.1007/s40501-016-0075-8.
Sackeim, H. (2003). Electroconvulsive therapy and schizophrenia. Schizophrenia, 517–551. https://doi.org/10.1002/9780470987353.ch27.
Salzman, C. (1980). The use of ECT in the treatment of schizophrenia. The American journal of psychiatry., 137, 1032–1041. https://doi.org/10.1176/ajp.137.9.1032.
Sanghani, S. N., Petrides, G., & Kellner, C. H. (2018). Electroconvulsive therapy (ECT) in schizophrenia: A review of recent literature. Current Opinion in Psychiatry, 31(3), 213–222. https://doi.org/10.1097/yco.0000000000000418.
Seethalakshmi, R., Parkar, S., Nair, N., & Pandit, A. (2006). Increased Thalamostriatal-Mesiotemporal glucose uptake with symptom remission in Schizophrenia-a case report. The Journal of ECT, 22(1), 74–75. https://doi.org/10.1097/00124509-200603000-00017.
Serafini, G., Pompili, M., Haghighat, R., Pucci, D., Pastina, M., Lester, D., Angeletti, G., Tatarelli, R., & Girardi, P. (2011). Stigmatization of schizophrenia as perceived by nurses, medical doctors, medical students and patients. Journal of Psychiatric and Mental Health Nursing, 18(7), 576–585.
Shapira, B., Tubi, N., Drexler, H., Lidsky, D., Calev, A., & Lerer, B. (1998). Cost and benefit in the choice of ECT schedule. The British Journal of Psychiatry, 172(1), 44–48. https://doi.org/10.1192/bjp.172.1.44.
Sinclair, D. J., Zhao, S., Qi, F., Nyakyoma, K., Kwong, J. S., & Adams, C. E. (2019). Electroconvulsive therapy for treatment-resistant schizophrenia. Cochrane Database of Systematic Reviews, 3. https://doi.org/10.1002/14651858.CD011847.pub2.
Tang, W.-K., & Ungvari, G. S. (2001). Rehab rounds: Electroconvulsive therapy in rehabilitation: The Hong Kong experience. Psychiatric Services, 52(3), 303–306. https://doi.org/10.1176/appi.ps.52.3.303.
Tang, W.-K., & Ungvari, G. S. (2002). Efficacy of electroconvulsive therapy combined with antipsychotic medication in treatment-resistant schizophrenia: A prospective, open trial. The Journal of ECT, 18(2), 90–94. https://doi.org/10.1097/00124509-200206000-00005.
Tang, W.-K., & Ungvari, G. S. (2003). Efficacy of electroconvulsive therapy in treatment-resistant schizophrenia: A prospective open trial. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 27(3), 373–379. https://doi.org/10.1016/s0278-5846(02)00354-8.
Tharyan, P., & Adams, C. E. (2005). Electroconvulsive therapy for schizophrenia. Cochrane Database of Systematic Reviews, 2. https://doi.org/10.1002/14651858.CD000076.pub2.
The U. K. (2003). Efficacy and safety of electroconvulsive therapy in depressive disorders: A systematic review and meta-analysis. The Lancet, 361(9360), 799–808. https://doi.org/10.1016/s0140-6736(03)12705-5
Üçok, A., & Çakir, S. (2006). Electroconvulsive therapy in first-episode schizophrenia. The Journal of ECT, 22(1), 38–42. https://doi.org/10.1097/00124509-200603000-00008.
Üçok, A., Çakir, S., Uçok, A., & Cakir, S. (2006). Electroconvulsive therapy in first-episode schizophrenia. Journal of ECT, 22(1), 38–42.
Usta Saglam, N. G., Aksoy Poyraz, C., Yalcin, M., & Balcioglu, I. (2020). ECT augmentation of antipsychotics in severely ill schizophrenia: A naturalistic, observational study. International Journal of Psychiatry in Clinical Practice, 24(4), 392–397. https://doi.org/10.1080/13651501.2020.1777313.
Ward, H. B., Szabo, S. T., & Rakesh, G. (2018). Maintenance ECT in schizophrenia: A systematic review. Psychiatry Research, 264, 131–142. https://doi.org/10.1016/j.psychres.2018.03.033.
Weickert, C. S., Weickert, T. W., Pillai, A., & Buckley, P. F. (2013). Biomarkers in schizophrenia: A brief conceptual consideration. Disease Markers, 35(1), 3–9. https://doi.org/10.1155/2013/510402.
Xiang, Y. T., Ungvari, G. S., Correll, C. U., Chiu, H. F., Lai, K. Y., Wang, C. Y., Si, T. M., Lee, E. H., He, Y. L., & Yang, S. Y. (2015). Use of electroconvulsive therapy for Asian patients with schizophrenia (2001–2009): Trends and correlates. Psychiatry and Clinical Neurosciences, 69(8), 489–496. https://doi.org/10.1111/pcn.12283.
Zhang, Z.-J., Chen, Y.-C., Wang, H.-N., Wang, H.-H., Xue, Y.-Y., Feng, S.-F., & Tan, Q.-R. (2012). Electroconvulsive therapy improves antipsychotic and somnographic responses in adolescents with first-episode psychosis—A case–control study. Schizophrenia Research, 137(1–3), 97–103.
Zheng, W., Cao, X.-L., Ungvari, G. S., Xiang, Y.-Q., Guo, T., Liu, Z.-R., Wang, Y.-Y., Forester, B. P., Seiner, S. J., & Xiang, Y.-T. (2016). Electroconvulsive therapy added to non-clozapine antipsychotic medication for treatment resistant schizophrenia: Meta-analysis of randomized controlled trials. PLoS One, 11(6), e0156510. https://doi.org/10.1371/journal.pone.0156510.
Zheng, W., Xiang, Y.-T., Tang, Y.-L., Xiang, Y.-Q., Li, X.-B., Cao, X.-L., Guo, T., Liu, Z.-R., Chiu, H. F., & Ungvari, G. S. (2019). Adjunctive electroconvulsive therapy for Schizophrenia: A meta-analysis of randomized rater-masked controlled trials [RETRACTED]. The Journal of ECT. https://doi.org/10.1097/yct.0000000000000344, Publish Ahead of Print.
Acknowledgments
Dr. Marco Zierhut is participant in the BIH-Charité Junior Clinician Scientist Program funded by the Charité –Universitätsmedizin Berlin and the Berlin Institute of Health. The Program was initiated and lead by Professor Duska Dragun to enable resident physicians to pursue a career in academic medicine. Professor Dragun passed away on December 28th of 2020. This publication is dedicated to her memory as a mentor, role model and stelar scientist.
Availability of Data and Material
All data and materials support the published claims and comply with field standards.
Code Availability
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the review. Marco Zierhut and Malek Bajbouj conventionalized the article, Marco Zierhut and Renaldo Bernard devised the methodology, Marco Zierhut, Eleanor Turner and Renaldo Bernard performed the literature search, data analysis and drafted the work, and Eric Hahn, Malek Bajbouj and Renaldo Bernard critically revised various drafts of the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
The authors declare that they have no conflict of interest.
Ethics Approval
The manuscript does not contain clinical studies or patient data.
Consent to Participate
All authors consent to participate.
Consent for Publication
All authors consent for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zierhut, M.M., Bernard, R.M., Turner, E. et al. Electroconvulsive therapy for negative symptoms in schizophrenia: a literature review from 2000 to 2021. Curr Psychol 42, 7512–7533 (2023). https://doi.org/10.1007/s12144-021-01989-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12144-021-01989-w