Abstract
In African American and Caucasian populations advanced age and preexisting co-morbid risk factors such as hypertension and diabetes have differential effects on the outcome of clinical complications such as systemic inflammatory response syndrome (SIRS) and sepsis when elderly patients encounter trauma/injury insult. Worldwide, the life span of the human population is increasing and the ageing population may become more susceptible to systemic inflammation and infection. Understanding of the complexities and characteristics specific to post-trauma clinical complications in the trauma care setting might help in designing strategies to improve outcomes and lead to the development of efficient patient care and management, particularly reducing long-term patient hospitalization which is a known risk factor for the development of infection in the elderly population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ageing in general is associated with a decline in health as well as host defense to environmental insults. Elderly individuals are at higher risk for infection and have often demonstrated increased susceptibility to systemic inflammatory response syndrome (SIRS) and sepsis after a traumatic injury that requires hospitalization (Shinoda-Tagawa and Clark 2003; Gomez et al. 2008). Despite more aggressive treatment-strategies some elderly patients require longer hospitalization which increases their exposure to pathogenic microbes (Roth et al. 2001; National Trauma Data Bank Annual Report 2007). Worldwide, sepsis accounts for about 20 million cases every year. In the USA it affects approximately 750,000 people and accounts for 215,000 deaths per year (Stüber et al. 1996; Holmes et al. 2003). According to the database from National Center for Injury Prevention and Control, >21.9 million people had traumatic injury in 2008, and roughly 15 % individuals were ≥65 years of age (Peschman et al. 2011). Ageing populations are often targets of infection induced clinical complication such as sepsis, an important cause of morbidity and mortality among the elderly, particularly after severe trauma and penetrating injury (Roth et al. 2001; Peschman et al. 2011). However, many traumatically injured patients have exhibited varying degree of systemic inflammation ranging from no significant clinical effects to SIRS, sepsis and organ failure leading to death. This is due to the fact that older adults display a broad range of variations in immune defense mechanisms resulting from impairment or a decline in immunologic parameters with age (Katz et al. 2004; Gomez et al. 2008).
In addition to old age, surgical procedures and anesthesia that some trauma/injured patients require has impact on immune modulation, affecting the innate inflammatory response to the insult (Lewis et al. 2007). Fundamentally, inflammatory response is a host defense response. Occasionally this response may cause deterioration in the condition of the host depending on endogenous and exogenous factors. Recent evidence supports the impact of ageing on innate immune response, which is essential for the development of adaptive immunity and defense against environmental factors (Katz et al. 2004; Panda et al. 2009).
Furthermore, evolutionarily conserved proteins such as Toll-like receptors (TLRs) that have been implicated in the regulation of inflammation were recently discovered to be associated with the development of infection and sepsis. These molecules are known for their ability to recognize and respond not only the distinct microbial structures [pathogen-associated- molecular patterns (PAMPs)], they also recognize endogenous non-microbial patterns [damage-associated molecular patterns (DAMPs)] that are released from human cells by injury, disease and old age (Modlin 2001; Leventhal and Schröppel 2012).
The underlying molecular causes of age-dependent immunologic changes have not been fully understood. However, evidence suggested that human leukocytes responsible for inflammatory responses may undergo significant changes during cell injury or cell death caused by disease, trauma, or old age which may affect the regulation and induction of inflammatory responses important for controlling microbial pathogens (Panda et al. 2009; Desai et al. 2010). Elderly individuals have shown susceptibility to many kinds of diseases including autoimmune diseases, vasculopathy, non-insulin dependent diabetes, and infection. In particular, it has been shown that diabetic patients with post-traumatic injuries that require longer hospitalization are more likely to develop infection and sepsis than those who did not have diabetes (Kao et al. 2006; Ahmad et al. 2007).
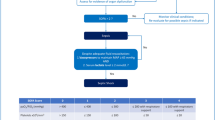
There have been multiple hospital-based studies, using trauma databases to investigate the impact of co-morbidity risk factors in association with injury severity score (ISS) and sepsis (Martin et al. 2003; Lazarus et al. 2005; Bochicchio et al. 2006). Although ISS is the gold standard for predicting the outcome of trauma, the values may be controversial in elderly patients (Roth et al. 2001; Bochicchio et al. 2006). A common speculation for increased morbidity and mortality in elderly patients with trauma would be a preexisting health disparity such as cardiovascular disease or diabetes (Berry et al. 2012). Limited studies have reported the impact of racial differences in infection and chronic disease such as diabetes and hypertension in the incidence of sepsis (Hollis et al. 2006; Dombrovskiy et al. 2007; Barnato et al. 2008; Redmond et al. 2011). Increasing evidence suggests that hypertension might be an immunologic disease. Thus, in instances of trauma, where alteration in immunologic response occurs, individuals with hypertensive vasculature may exhibit a stronger response leading to tissue damage and the development of systemic inflammatory response (Dörffel et al. 1999; Harrison et al. 2008). A diagrammatic hypothetical illustration of the impact of trauma, stress and age is given in Fig. 1.
This is a hypothetical model of trauma induced and age related tissue damage leading to inflammation and cytokine production. A series of events following tissue injury by trauma or ageing occurs, causing activation of inflammatory immune system. Upon interaction between TLRs (located on macrophages and dendritic cells) and endogenous molecules or tissue debris released from damaged-cells, an intracellular signaling cascade becomes activated, initiating cytokine expression and release. Over- production of cytokines either causes an immunologic imbalance or paralysis of the immune system which may lead to systemic inflammation, sepsis, multiple organ dysfunction and death.
Due to the worldwide rise in the elderly population, most studies have focused on health and socioeconomic issues reflecting the elderly wellbeing in the society (Carr 2009; Ghazi-Tabatabaei and Karimi 2011; Johnson and Duraiswamy 2011; Akushevich et al. 2012). The primary goal of our study has been the development of a screening tool for early identification of patients undergoing post-traumatic injury complication with characteristics of SIRS and or sepsis. The objective of this study is to investigate if advanced age increases the likelihood of developing SIRS and/or sepsis after a traumatic injury in addition to the number of insults or the ISS and health disparities such as diabetes and hypertension. Furthermore, this study is designed to compare the differences in the outcomes in African American vs. Caucasian patients in the surgical intensive care unit (SICU). Improvement in management of clinical complications in elderly patients in the critical care settings may help the overall patient wellbeing and prevent the need to go to a long-term care facility which has greater risk of morbidity and mortality.
Materials and Methods
Data Collection and Clinical Characteristics
Charts of a total of 800 trauma patients, who were admitted to the surgical intensive care unit (SICU) at the University of Mississippi Medical Center (UMMC) between 2006 and mid-2008 were reviewed. Only the 546 patients whose charts contained an adequate database for this study were included. Health disparities including co-morbid medical conditions such as diabetes and hypertension were investigated. The criterion for inclusion and exclusion is presented in Table 1. Patient’s Injury severity score (ISS) is calculated based on the patient’s anatomical and physiological status when the patient arrives to the SICU. The ISS is a commonly used numerical system that provides a reliable estimation of patient status and survival. It is correlated linearly with clinical outcomes including hospital and other measures of severity of patient’s condition. For example, an abdominal injury with ruptured spleen represents an ISS = of 25. Fractured femur or a single rib each represent an ISS = of 3.
Two hundred fifty four patients with incomplete clinical data relevant to this study were excluded. We have used generalized standard criteria for determination of SIRS and sepsis. Patients were considered septic when they had two or more of the clinical signs of SIRS, such as temperature >38 °C or <36 °C; heart rate >90 bpm or tachycardia; respiratory rate >20 breaths/min, PaCO2 <32 or hyperventilation; white blood cell counts (WBC) >12,000/mL or <4,000/mL or presence of >10 % immature neutrophils and a positive culture for microbial infection. However, since some patients were required to use the ventilator, we excluded respiratory rate (RR) values for those patients from the list of clinical signs for SIRS. But we included organ failure as one of the determining factors if RR was not useful.
Statistical Analysis
Patient’s health disparity data, age, race, gender and presenting ISS were analyzed using a SAS program to determine the effect on the clinical outcomes. A logistic regression model was used for predication of probability of occurrence of SIRS or sepsis on the bases of ISS or age, and in the context of possible confounders in relation to clinical outcomes. A p-value of <0.05 was used for all statistics. P-values were computed from Cochran-Mantel-Haenszel statistics and adjusted odds ratios were logistic regression.
Results
Global Analysis of Demographic and Co-Morbidity Factors
A total of 546 patients, 261 African American and 285 Caucasian were included in the data analysis. The remaining patients were excluded due to insufficient data. Health disparity data (HDD) including diabetes and hypertension were assessed. Other health disparities such as chronic heart, pulmonary or kidney diseases that previously were reported to have impact on mortality rates were not included in this study.
For the analysis of frequency distribution of demographic, trauma factors and co-morbid medical conditions, patients were stratified based on gender and race into the following three age ranges: <35, 35–64, and 65–100 (Table 2). In an overall analysis the percentage of patients that exhibited complication free status at post-trauma/injury decreased with age (no complication %: age <35, 77.7 %; age 35–64, 47.5 % and age 65–100, 2.4 %, p < 0.00001, odds ratio =143.5). There was a statistically significant difference between the two groups, particularly when comparing females and males separately. Hypertension (HTN) was increased 2.5–3.6 fold in African American female patients, age <35 than in all other patients in the same age group [(p < 0.03, Odds Ratio (OR) =4.7)]. The incidence of motor vehicle collision (MVC) was highest in Caucasian females, age <35 and Caucasian males, age 65–100 (p < 0.0001). The incidence of gunshot wound (GSW) was higher in African American patients age 35–64 as compared with both male and female groups (p < 0.0001). MVC was increased a 2.4 fold among African American male, age 65–100, OR = 7.5, and Fall/others was highest in African American female and lowest in Caucasian male, age 65–100, OR = 5.0.
Post-Trauma Induced Clinical Complications Stratified by Race, ISS and Age
During the first global analysis of the data, the presenting ISS had a statistically significant impact on the development of SIRS and sepsis (p < 0.0001). After separating the study subjects by race and age the ISS remained a significant factor in the development of SIRS and sepsis for both African American and Caucasian patients (p < 0.0009 to <0.0001), (Table 3). We also observed that age in African American patients may have played a significant role in the development of both SIRS (p < 0.04) and sepsis (p < 0.03). African American patients <35 years of age were significantly less susceptible to the development of sepsis as compared with patients age between 65 and100 years of age (p < 0.03). Such incidence was not observed in Caucasian patients (Table 3).
Health Disparity in the Context of Stratified ISS and Age
Using a multivariate logistic regression model analysis, including injury severity scores (ISS < 15, ISS = 15–28 and ≥29), and health disparity (DM and HTN), significant associations were observed in both African American and Caucasian groups comparing patients at age <65 vs. ≥65. Data is presented in Figs. 2 and 3 for African American and Caucasian patients respectively. In African American patients multiple risk factors variably contributed to the clinical complications in elderly patients aged 65–100. Each risk factor was evaluated for its potential impact on the outcome of SIRS alone and or sepsis. HTN was an independent risk factor associated with SIRS and/or sepsis in elderly patients with a minimal injury score of <15, (p < 0.007 and p < 0.05 respectively) as compared with patients with a moderate to severe level of injury. In contrast patients age <65, with pre-existing HTN were more susceptible to the development of SIRS and sepsis when their injury level was more than the minimal (ISS = 15–28) as compared with other groups (p < 0.05). While pre-existing diabetes (DM) had impact on susceptibility for SIRS and sepsis at all three categories of ISS in elderly patients, it was only statistically significant when the ISS was ≥29 (p < 0.05 and p < 0.01 respectively).
In Caucasians, (Fig. 3) patients age ≥65 with a history of diabetes and/or HTN were at higher risk of developing SIRS as compared with others when the ISS was between 15 and 28 (p < 0.05 and 0.009 respectively). It should be noted that we observed a higher percentage of SIRS and sepsis in elderly patients with diabetes and HTN when the ISS was ≥29, but the difference was not statistically significant.
Post-Trauma/Injury Clinical Complications in Elderly Patients
It was observed that an advanced age (65–100) had an impact on clinical complications such as SIRS and or sepsis. Therefore, we next stratified the data based on gender, race and ISS in elderly patients age 65–100. As shown in Fig. 4, the frequency distribution of the incidence of SIRS was increased 1.6-fold in Caucasian male patients, age ≥65 who had suffered intermediate levels of injury (ISS = 15–28) as compared with African American male patients in the same ISS range (P < 0.05). In contrast, both African American and Caucasian female patients presented with a higher percent of SIRS, when the ISS was ≥29 as compared with male patients in the same ISS range (p < 0.05). However, the Caucasian female patients presented with the lowest sepsis when the injury score was ≥29 as compared with other groups (p < 0.04). The incidence of sepsis was increased 2.4-fold in African American female patients age ≥65 with either minimal ISS or intermediate levels of injury (ISS = 15–28), as compared with Caucasian females with the same ISS range (sepsis %: 55.5 % vs. 23 % respectively, p < 0.045). The episodes of sepsis were lowest for Caucasian females in all 3 categories of ISS, indicating that Caucasian females age ≥65 have the least risk of developing sepsis regardless of the intensity of the injury as compared with others in the same study category (p < 0.04). The underlying mechanisms of such differences require further investigation.
Post trauma/injury clinical complication such as SIRS and Sepsis was stratified by gender race and ISS in elderly patients, ≥ 65 years of age. ISS was influential in the outcome of sepsis in both African American and Caucasian patients. However, lower ISS was influential in the development of SIRS only in male patients
Discussion
We have tested the hypothesis that an advanced age and health disparities such as diabetes and hypertension have different impacts on post-trauma/injury clinical outcomes in African American patients as compared with Caucasians in the SICU. The evidence for the effects of co-morbid risk factors such as hypertension and diabetes were more prominent at an older age in African American patients vs. Caucasians. These risk factors were associated with higher incidence of SIRS and sepsis after trauma/injury insults. Worldwide, the life span of the human population is increasing and the ageing population may become more susceptible to systemic and chronic diseases. In U.S. elderly patients (≥ 65 years of age) are accounted for >12 % of population and 64.9 % of sepsis cases as compared with younger patients (Martin et al. 2006), thus, more preventive measures will be needed to reduce long-term patient care. The length of hospitalization has been a known risk factor for the development of infection particularly in elderly patients (Shinoda-Tagawa and Clark 2003; Akushevich et al. 2012). A number of studies have investigated the management of coronary artery diseases, end-stage kidney and pulmonary diseases, and trauma induced clinical complications in the general population. They have shown that such clinical conditions have a drastic impact on the outcome of post-trauma clinical complications, particularly, the length of hospitalization and mortality rates (Hoste et al. 2003; Merx and Weber 2007; Zanotti-Cavazzoni and Hollenberg 2009). In addition, it has previously been reported that preexisting medical conditions have the largest effect on middle aged patients with minor to moderate injuries (Victorino et al. 2003; McGwin et al. 2004). However, the differences in age and preexisting health disparities on the outcome of SIRS and sepsis have not been explored in the context of ethnicity. In traumatic injury settings, ascertainment of preexisting health information is difficult, since medical records are not always adequately documented, and the information obtained from relatives may not be reliable. Such issues in retrospective studies have been discussed previously by other investigators (Victorino et al. 2003; McGwin et al. 2004). In our study, more than 30 % of the reviewed charts were excluded because of poor quality data ascertainment. We assessed the potential influence of co-morbid factors that may have an impact on SIRS and sepsis after a range of traumatic injuries in elderly patients ≥65, in the context of age, race and gender.
Overall, in a multivariate analysis, adjusting for the ISS and age, old age was an important risk factor in the development of systemic inflammation and sepsis in African American patients (p < 0.03). However, univariate analysis of the data showed additional risk factors, particularly in African American trauma patients. HTN and diabetes were variable risk factors regardless of the ISS. These findings indicated that the ISS may not fully influence the outcome of sepsis, and patients susceptible to the development of sepsis were a unique subset with a potential for infection. Nonetheless, considering the impact of ISS and age demonstrated by others (Dekeyser et al. 2002; Martin et al. 2006; Ong et al. 2009), as well as in this study, the ISS and age may be the primary risk factors, and the other factors, such as diabetes and HTN might have additive effects in the development of clinical outcomes. Although pre-existing health status was influential in the outcomes, it was not possible to determine in our study. Thus, in the context of this study, to get a better estimate of the impact of pre-existing health conditions, a much larger study will have to be conducted.
Regarding HTN, studies have shown that the inter-individual differences in blood pressure are under genetic control and particularly, in African American population, the physiologic system that controls salt retention is influenced by gene variation (Jain et al. 2002; Hopkins et al.; 2002; Johnson et al. 2009). There is ample evidence demonstrating the gene-host and gene-environment interactions that play a role in the morbidity and mortality associated with post-trauma induced complications (Fernández-Morera et al. 2010; Hildebrand et al. 2011). In our study the impact of HTN exhibited a variable outcome in the context of age and ISS. African American patients age ≥65 with HTN presented with a higher percentage of SIRS and sepsis despite minimal injury (ISS < 15) as compared with those patients age <65 (p < 0.007, p < 0.05, respectively). In contrast, elderly patients with intermediate level of injury (ISS = 15–28) were less susceptible to SIRS and sepsis as compared with patients age <65. This indicates that older patients with HTN are more predisposed to vascular tissue damage due to their disease condition and a minimal trauma may trigger a robust proinflammatory response making them more likely to develop sepsis. The younger patients appeared to recover from SIRS when there was minimal trauma, and they did not develop sepsis. Caucasian patients age ≥65, with HTN also showed an increased susceptibility to sepsis as compared with those age <65. However, our data was statistically not significant. This disparity in result is more likely related to multiple factors that ultimately affect the outcome of sepsis. Variation in the etiology of microbial pathogens may cause differences in the recovery outcome. Genetic studies support the potential for the unequal distribution of disease markers associated with complex disorders such as HTN in different populations (Kunes and Zicha 2009; Wei et al. 2011). In addition, many genetic markers including cytokine and human leukocyte antigen (HLA) gene polymorphisms that have been associated with inflammation-based diseases or infection pathways have been found more frequently in African Americans than in Caucasians (Ness et al. 2004; Tchernitchko et al. 2005). Tissue associated antigens including HLA and TLR molecules that are influential in antigen recognition and the clearance of pathogenic microorganisms have been shown to decline with age (Boehmer et al. 2004) causing leukocyte dysfunction and reduction in defense mechanism. The decline in the defense mechanism with old age may vary among the population. Furthermore, the magnitude of health disparity plays a crucial role in defense mechanism in the ageing population. Racial and ethnic disparities in health have been discussed in many studies (Liang et al. 2010; Sarkin et al. 2013; Zhivan et al. 2012). The underlying mechanisms for decline in defense mechanisms with age include defects in immunity known as “inflamm-ageing” associated with disability and impairment to repair. In addition, poor medical decision making and limited access to medical care are potential risk factors in the development of poor immune system in elderly individuals.
We also addressed the role of diabetes in the ageing population in trauma induced clinical complications. In both African American and Caucasian populations, elderly patients (age ≥65) demonstrated increased rates of SIRS and sepsis if they had diabetes. However, in African American patients the impact was statistically significant when the ISS injury was ≥29, whereas, in Caucasian patients the impact was statistically significant when injury was less severe (ISS = 15 and 28). Although diabetes in African American adults is influenced by the same risk factors that are associated with diabetes in Caucasians; increasing evidence supports the influence of non-diabetes genetic markers that are differentially expressed in African American diabetics vs. Caucasian diabetics. A recent study has shown that African American patients with Type 2 diabetes had 2–6 times more altered genes than Caucasians patients with diabetes (Mao et al. 2011). In the same study, gene expression profiling data demonstrated a significant variation in the signaling pathways associated with innate and adaptive immune response cells in African American vs. Caucasian patients with Type 2 diabetes. Such variation in immune response genes might be influential in the development of clinical complications after trauma/injury particularly in ageing individuals. Elderly patients with diabetes often present with chronic inflammation. Therefore such patients may respond to a minimal trauma insult rapidly, while some may also recover from subsequent infection and become less susceptible to sepsis. However, the same immune system may not function adequately against severe injury which may lead to the development of sepsis. Elderly Caucasian patients in this study were more susceptible to SIRS and sepsis when injury was intermediate (ISS = 15–28), but, the incidence of SIRS and sepsis was increased significantly with severe injury in African American patients. We note that in our study, more African American patients had Type 2 diabetes than Caucasian patients. Epidemiologic evidence shows that African Americans tend to be more insulin resistant (Type 2) and have greater insulin response to glucose than Caucasians (Osei and Schuster 1996; Gower et al. 2002; Cheng et al. 2012). The difference in the type of diabetes may correlate with the susceptibility outcome observed in this study. One might also speculate that in elderly Caucasian patients, the severity of the injury (ISS ≥ 29) could have a higher impact on the overall pathophysiology and may overwhelm the contribution of preexisting diabetes.
These findings agree with other reports in the medical literature. Hollis et al. 2006, have demonstrated significant increases in mortality rates in patients with preexisting medical conditions (PMC), who had low to moderate ISS. McGwin et al. 2004, have also shown the adverse effects of severity on the risk of mortality in patients with chronic medical conditions (CMCs). In fact, it was shown that multiple preexisting health conditions become a higher mortality risk category despite minor to moderate injury rates in elderly patients. Our study was in concordance with previous reports in both African American and Caucasian patients (Table 2), but the end point of our study was an observation of SIRS and/or sepsis and not mortality.
The impact of age on sepsis was clearly demonstrated in African American patients but not in Caucasian patients. Furthermore, older African American patients (≥ 65 years of age) who developed SIRS were at a 1.4-fold higher risk of developing sepsis as compared with patients age <65. The contribution of differences in lifestyle or environmental factors cannot be ruled out in this study. Current health education approach indicates that more health associated information patients have, the better they manage their illness. Demographic variables such as education levels and ethnicity play significant role for seeking medical information in the elderly people (Rooks et al. 2012). In addition, older patients with mobility impairments whom require caregiver and home health care, continuously are at higher risk of developing common illnesses which diminishes not only the quality of life for them, it reduces the normal immunity to the pathogens. The protection from sepsis in younger patients may result from stability of the individual’s immunologic balance that are in part due to the age regulated genes or from the absence of the age related health disparity in these patients.
It is known that the immune system gradually undergoes changes due to ageing that affects both the innate and adaptive immunity (Chung et al. 2009). A result of such changes is the dysregulation of the immune system and an increase in SIRS arising from the release of higher amounts of inflammatory cytokines upon exposure to both internal and external insults. In addition, in some instances a state of low-grade chronic inflammation referred to as “inflamm-ageing” occurs after age 40–50 (Franceschi 2007) which might provide an excellent environment for the development and persistence of infection. Our data on the age association of sepsis in African American patients supports an important observation regarding race and infection. A direct relevance with regard to age and infection could be the role of Toll-like receptors (TLRs) in recognizing the pathogenic microorgansms. TLRs are expressed on a variety of immune response cells, particularly monocytes and macrophages. Through the signaling by TLRs and monocyte co-stimulatory factors the pathogenic microorganisms are phagocytized and eliminated from the body. It has been shown that TLR-induced signaling is reduced significantly in the elderly (Renshaw et al. 2002; van Duin and Shaw 2007), which could influence the impaired phagocytosis of microorganism. It was reported by others and us that in African American patients with sepsis, polymorphisms of TLR-2 and TLR-4 genes were associated with increased susceptibility to infections and increased incidence of sepsis in SICU patients (Agnese and Calvano 2002; McDaniel et al. 2007). Such variation in gene expression might correlate with our findings that older African American patients were more susceptible to developing sepsis.
In summary this study highlighted that the association of preexisting risk factors have differential effects on the outcome of clinical complications after trauma/injury insults in elderly African American vs. Caucasian populations. Caucasian male patients age ≥65 were at higher risk of developing SIRS with an intermediate level of injury, than the others of the same age group. Caucasian female patients age ≥65 presented with the lowest episodes of sepsis regardless of ISS as compared with others of the same age group. Given the complexity of sepsis, it is more likely that a combination of epigenetics, preexisting health and social disparity are confounding factors for diagnosis and prognosis of sepsis, and identification of such factors might have a significant survival benefit after trauma injury in the elderly population.
References
Agnese, D. M., & Calvano, J. E. (2002). Human Toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. Journal of Infectious Disease, 186, 1522–1525.
Ahmad, R., Cherry, R. A., Lendel, I., Mauger, D. T., Service, S. L., Texter, L. J., et al. (2007). Increased hospital morbidity among trauma patients with diabetes mellitus compared with age and injury severity score-matched control subjects. Archives of Surgery, 142(7), 613–618.
Akushevich, I., Kravchenko, J., Ukraintseva, S., Arbeev, K., & Yashin, A. I. (2012). Age patterns of incidence of geriatric disease in the U.S. Elderly population: medicare-based analysis. Journal of the American Geriatrics Society, 60, 323–327.
Barnato, A. E., Alexander, S. L., Linde-Zwirble, W. T., & Angus, D. C. (2008). Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. American Journal of Respiratory and Critical Care Medicine, 177(3), 279–284.
Berry, J. D., Dyer, A., Cai, X., Garside, D. B., Ning, H., Thomas, A., et al. (2012). Lifetime risks of cardiovascular disease. New England Journal of Medicine, 366(4), 321–328.
Bochicchio, G. V., Joshi, M., Bochicchio, K., Shih, D., Meyer, W., & Scalea, T. M. (2006). Incidence and impact of risk factors in critically ill trauma patients. World Journal of Surgery, 30(1), 114–118.
Boehmer, E. D., Goral, J., Faunce, D. E., & Kovacs, E. J. (2004). Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. Journal of Leukocyte Biology, 75(2), 342–349.
Carr, D. (2009). Aging in America: the link between productivity and resources in the third age. Ageing International, 34, 154–171.
Cheng, C.-Y., Reich, D., Haiman, C. A., Tandon, A., Patterson, N., Elizabeth, S., et al. (2012). African ancestry and its correlation to type 2 diabetes in African Americans: a genetic admixture analysis in three U.S. Population cohorts. PLoS ONE, 7(3), e32840. doi:10.1371/journal.pone.0032840.
Chung, H. Y., Cesari, M., Anton, S., Marzetti, E., Giovannini, S., Seo, A. Y., et al. (2009). Molecular inflammation: underpinnings of aging and age-related diseases. Aging Research Reviews, 8, 18–30.
DeKeyser, F., Avitzour, M., Watts, D. D., Trask, A. L., & Muggia-Sullam, M. (2002). International trauma care: a comparison between Jerusalem, Israel and Fairfax county, Virginia, USA. The Israel Medical Association Journal, 4(2), 103–108.
Desai, A., Grolleau-Julius, A., & Yung, R. (2010). Leukocyte function in the aging immune system. Journal of Leukocyte Biology, 87, 1001–1009.
Dombrovskiy, V. Y., Martin, A. A., Sunderam, J., & Paz, H. L. (2007). Occurrence and outcomes of sepsis: influence of race. Critical Care Medicine, 35(3), 763–768.
Dörffel, Y., Lätsch, C., Stuhlmüller, B., Schreiber, S., Scholze, S., Burmesterr, G. R., et al. (1999). Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension, 34(1), 113–117.
Fernández-Morera, J. L., Calvanese, V., Rodrígues-Rodero, S., Menéndez-Torre, E., & Fraga, M. F. (2010). Epigenetic regulation of the immune system in health and disease. Tissue Antigens, 76, 431–439.
Franceschi, C. (2007). Inflammaging as a major characteristic of old people. Can it be presented or cured? Nutrition Reviews, 65(12), S173–S176.
Ghazi-Tabatabaei, M., & Karimi, S. (2011). Socio-demographic economic and structural correlates of intergeneration support of the elderly in Iran. Ageing International, 36, 428–444.
Gomez, C. R., Nomellini, V., Faunce, D. E., & Kovacs, E. J. (2008). Innate immunity and aging. Experimental Gerontology, 43(8), 718–728.
Gower, B. A., Granger, W. M., Franklin, F., Shewchuk, R. M., & Goran, M. I. (2002). Contribution of insulin secretion and clearance to glucose-induced insulin concentration in African American and Caucasian children. Journal of Clinical Endocrinology and Metabolism, 87, 2218–2224.
Harrison, D. G., Guzik, T. J., Goronzy, J., & Weyand, C. (2008). Is hypertension an immunologic disease? Current Cardiology Reports, 10(6), 464–469.
Hildebrand, F., Mommsen, P., Frink, M., van Griensven, M., & Kretteck, C. (2011). Genetic predisposition for development of complications in multiple trauma patients. Shock, 35(5), 440–448.
Hollis, S., Lecky, F., Yates, D. W., & Woodford, M. (2006). The effect of pre-existing medical conditions and age on mortality after injury. Journal of Trauma, 61(5), 1255–1260.
Holmes, C. L., Russell, J. A., & Walley, K. R. (2003). Genetic polymorphisms in sepsis and septic shock. Role in prognosis and potential for therapy. Chest, 124(3), 1103–1115.
Hopkins, P. N., Hunt, S. C., Jeunemaitre, X., Smith, B., Solorio, D., Naomi, D. L., et al. (2002). Angiotensinogen genotype affects renal and adrenal responses to angiotensin II in essential hypertension. Circulation, 105, 1921–1927.
Hoste, E. A. J., Lameire, N. H., Vanholder, R. C., Benoit, D. D., Decruyenaere, J. M. A., & Colardyn, F. A. (2003). Acute renal failure in patients with sepsis in a surgical ICU. Predictive factors, incidence, comorbidity and outcome. Journal of the American Society of Nephrology, 14(4), 1022–1030.
Jain, S., Tang, X., Narayanan, C. S., Agarwal, Y., Peterson, S. M., Brown, C. D., et al. (2002). Angiotensinogen gene polymorphism at −217 affects basal promoter activity and is associated with hypertension in African Americans. Journal of Biological Chemistry, 277(39), 36889–36896.
Johnson, C. S., & Duraiswamy, M. (2011). Health service provider’s perspectives on healthy aging in India. Ageing International, 36, 445–462.
Johnson, A. D., Gong, Y., Wang, D., Langaee, T. Y., Shin, J., Cooper-Dehoff, R. M., et al. (2009). Promoter polymorphisms in ACE (angiotensin I-converting enzyme) associated with clinical outcomes in hypertension. Clinical Pharmacology & Therapeutics, 85(1), 36–44.
Kao, L. S., Todd, S. R., & Moore, F. A. (2006). The impact of diabetes on outcome in traumatically injured patients: an analysis of the National Trauma Data Bank. American Journal of Surgery, 192(6), 710–714.
Katz, J. M., Plowden, J., Renshaw-Hoelscher, M., Lu, X., Tumpey, T. M., & Sambhara, S. (2004). Immunity to influenza: the challenges of protecting an aging population. Immunologic Research, 29(1–3), 113–124.
Kunes, J., & Zicha, J. (2009). The interaction of genetic and environmental factors in the etiology of hypertension. Physiological Research, 58(Suppl 2), S33–S41.
Lazarus, H. M., Fox, J., Burke, J. P., Lioyd, J. F., Snow, G. L., Mehta, R. R., et al. (2005). Trauma patient hospital-associated infections, risks and outcomes. Journal of Trauma, 59(1), 188–194.
Leventhal, J. S., & Schröppel, B. (2012). Toll-like receptors in transplantation: sending and reacting to injury. Kidney International, 81, 826–832.
Lewis, M. C., Abouelenin, K., & Paniagua, M. (2007). Geriatric trauma: special considerations in the anesthetic management of the injured elderly patients. Anesthesiology Clinics, 25(1), 75–90.
Liang, J., Quinones, A. R., Bennett, J. M., Ye, W., Xu, X., Shaw, B. A., et al. (2010). Evolving self-related health in middle and old age: How does it differ across Black, Hispanic, and White Americans? Journal of Aging and Health, 22, 3–26.
Mao, J., Ai, J., Zhou, X., Shenwu, M., Ong, M., Blue, M., et al. (2011). Transcriptomic profiles of peripheral white blood cells in type II diabetes and racial differences in expression profiles. BMC Genomics, 12(suppl 5), S5–S12.
Martin, G. S., Mannino, D. M., Eaton, S., & Moss, M. (2003). The epidemiology of sepsis in the United States from 1979 through 2000. New England Journal of Medicine, 348(16), 1546–1554.
Martin, G. S., Mannino, D. M., & Moss, M. (2006). The effect of age on development and outcome of adult sepsis. Critical Care Medicine, 34(1), 15–21.
McDaniel, D. O., Hamilton, J., Brock, M., May, W., Calcote, L., Tee, L. Y., et al. (2007). Molecular analysis of inflammatory markers in trauma patients at risk of post injury complications. Journal of Trauma, 63(1), 147–158.
McGwin, G., Jr., MacLennan, P. A., Fife, J. B., Davis, G. G., & Rue, L. W., III. (2004). Pre-existing conditions and mortality in older trauma patients. Journal of Trauma, 56(6), 1291–1296.
Merx, M. W., & Weber, C. (2007). Sepsis and the heart. Circulation, 116(7), 793–802.
Modlin, R. L. (2001). Activation of Toll-like receptors by microbial lipoproteins: role in host defense. Journal of Allergy and Clinical Immunology, 108, S104–S106.
National Trauma Data Bank Annual Report. (2007). Available at: http://www.facs.org/trauma/ntdb/ntdbannualreport2007.pdf. [Accessed March 28, 2008].
Ness, R. B., Haggerty, C. L., Harger, G., & Ferrell, R. (2004). Differential distribution allelic variants in cytokine genes among African American and White American. American Journal of Epidemiology, 160(11), 1033–1038.
Ong, A. W., Omert, L. A., Vido, D., Goodman, B. M., Protetch, J., Rodriguez, A., et al. (2009). Characteristics and outcomes of trauma patients with ICU lengths of stay 30 days and greater: a seven-year retrospective study. Critical Care, 13(5), R154–R162.
Osei, K., & Schuster, D. P. (1996). Effects of race and ethnicity on insulin sensitivity, blood pressure, and heart rate in three ethnic populations. Comparative studies inn African-Americans, African Immigrants (Ghanaians), and White Americans using ambulatory blood pressure monitoring. American Journal of Hypertension, 9, 1157–1164.
Panda, A., Arjona, A., Sapey, E., Bai, F., Fikrig, E., Montgomery, R. R., et al. (2009). Human innate immunosenescence: causes and consequences for immunity in old age. Trends in Immunology, 3, 325–333.
Peschman, J., Neideen, T., & Brasel, K. (2011). The impact of discharging minimally injured trauma patient: does age play a role in trauma admission. Journal of Trauma, 70(6), 1331–1336.
Redmond, N., Baer, H. J., & Hicks, L. S. (2011). Health behaviors and racial disparity in blood pressure control in the national health and nutrition examination survey. Hypertension, 57, 383–389.
Renshaw, M., Rockwell, J., Engleman, C., Gewirtz, A., Katz, J., & Sambhara, S. (2002). Impaired Toll-like receptor expression and function in aging. Journal of Immunology, 169, 4697–4701.
Rooks, R. N., Wiltshire, J. C., Elder, K., Belue, R., & Gary, L. C. (2012). Health information seeking and use outside of the medical encounter: is it associated with race and ethnicity? Social Science and Medicine, 74, 176–184.
Roth, B. J., Velmahos, G. C., Order, D. B., Vassiliu, P., Tatevossian, R., Demetriades, D., et al. (2001). Penetrating trauma in patients older than 55 years: a case–control study. Injury, 32(7), 551–554.
Sarkin, A. J., Groessl, E. J., Mulligan, B., Sklar, M., Kaplan, R. M., & Ganiats, T. G. (2013). Racial differences in self-rated health diminishing from 1972 to 2008. Journal of Behavioral Medicine, 36(1), 44–50.
Shinoda-Tagawa, T., & Clark, D. E. (2003). Trends in hospitalization after injury: older women are displacing young men. Injury Prevention, 9, 214–219.
Stüber, F., Peterson, M., Bokelmann, F., & Schade, U. (1996). A genomic polymorphism within the tumor necrosis factor-a concentrations and outcome of patients with severe sepsis. Critical Care Medicine, 24(3), 381–384.
Tchernitchko, D., Chiminqgi, M., Galactéros, F., Prehu, C., Segbena, Y., Coulibaly, H., et al. (2005). Unexpected high frequency of P46L TNFRSF1A allele in sub-Saharan West African population. European Journal of Human Genetics, 13(4), 513–515.
van Duin, D., & Shaw, A. C. (2007). Toll-like receptors in older adults. Journal of American Geriatrics Society, 55, 143–1444.
Victorino, G. P., Chong, T. J., & Pal, J. D. (2003). Trauma in the elderly patients. Arch Surgery, 138, 1093–1098.
Wei, P., Milbauer, L. C., Enenstein, J., Nguyen, J., Pan, W., & Hebbel, R. P. (2011). Differential endothelial cell gene expression by African Americans versus Caucasian Americans: a possible contribution to health disparity in vascular disease and cancer. BMC Medicine, 9, 2–18.
Zanotti-Cavazzoni, S. L., & Hollenberg, S. M. (2009). Cardiac dysfunction in severe sepsis ad septic shock. Current Opinion in Critical Care, 15(5), 392–397.
Zhivan, N. A., Ang, A., Amaro, H., Vega, W. A., & Markides, K. S. (2012). Ethnic/race differences in the attrition of older American survey respondents: implications for health-related research. Health Services Research. doi:10.1111/j.1475-6773.2011.01322.x.
Acknowledgments
This study was funded in part by a Biomedical Research Grant from the UMMC. We thank Amber Kyle, RN, in the Trauma Division for providing access to trauma database and Dr. Larry McDaniel, in the Department of Microbiology and the Division of Infectious Diseases at UMMC for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McDaniel, D.O., Rigney, D., Olivier, J. et al. Trauma Induced Inflammation, Sepsis and Ageing. Ageing Int 39, 243–258 (2014). https://doi.org/10.1007/s12126-013-9195-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12126-013-9195-2