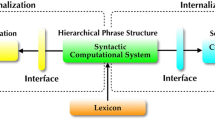
Abstract
The biological foundations of language reflect assumptions about the way language and biology relate to one another, and with the rise of biological studies of language, we appear to have come closer to a deep understanding of linguistic cognition–the part of cognition constituted by language. This article argues that relations of neurobiological and genetic instantiation between linguistic cognition and the underlying biological substrate are ultimately irrelevant to understanding the higher-level structure and form of language. Linguistic patterns and those that make up the character of cognition constituted by language do not simply arise from the biological substrate because higher-level structures typically assume forms based on constraints that only emerge once these new levels are constructed. The goal is not to show how the mapping problem between linguistic cognition and neurobiology can be solved. Rather, the goal is to show the mapping problem ceases to exist once a different understanding of language-(neuro)biology relations is embraced. With this goal, this article first uncovers a number of logical and conceptual fallacies in strategies deployed in understanding language-(neuro)biology relations. After having shown these flaws, the article offers an alternative view of language-biology relations that shows how biological constraints shape language (nature and form), making it what it is.



Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Notes
In this paper, ‘linguistic cognition’ is demarcated to include not just the form of cognitive representations formatted by natural language but also psycholinguistic processes or procedures that are used for language but are part of the ensemble of all cognitive processes the brain allows for.
The relation of supervening is an ontological relation specifying how a lower level/scale entity determines the form and organization of a higher level/scale entity. For instance, subjective states of the mind (such as feelings and emotions) can be said to supervene on the physical properties and processes in the brain because any changes in subjective states are due to the changes in the physical properties and processes in the brain. In the present case, this is about the relation of processes in the brain to the form of linguistic cognition. The supervening relation could tell us whether any differences in the form of linguistic cognition are due to changes in the physical states and processes in the brain. This can be partly constrained by the complexity of linguistic cognition (the whole) from the lower-level neurons and neuronal networks (the parts).
Although the system of language in the mind is attributed to speakers and listeners that operate under severe temporal constraints and constrains of working memory, there is no denying that it is the same system of language that also allows for the formation of an unbounded number of (or possibly infinite) constructions and expressions. It is this very fact that has led Chomsky (2000) to posit Merge as the fulcrum of both abstractions and its implementation in the brain–this seems to have confounded the distinction between the character of abstractions in a formal system and the nature of functioning of a finite system such as the brain (Postal 2009). Hence the problem of how the finite human mind/brain gives rise to potentially infinite linguistic structures does not arise for those who hold on to the Chomskyan conception. The present paper does not, of course, presuppose what the Chomskyan conception presupposes.
The concept of multiple realization is usually traced to Putnam (1967). A cognitive function is multiply realizable only if it can be instantiated in multiple types of substrate. In the present case, the human neuronal machinery is unique for the unique cognitive organization that language gives rise to.
Even when neural networks are said to learn from experience, or the brain is rewired to read words, the nature of the causal information flow from experiences to the brain is not fully understood (Rolls 2021). Besides, the causal influence of experiences is one thing, and the information flow in interactions between the brain and abstractions in language is another. The latter is a mind-dependent product of the former.
It may be noted that for Chomsky unification does not simply mean that the mental realm has to be united with the neurophysiological system because this tends to re-interpret the mental in terms of what is physical. Rather, he thinks the neurophysiological level, which is of course physical, may turn out to be mental at a lower level as we go about exploring the properties of the mind at various levels. Thus, unification in this sense does not presuppose a serious ontological distinction between the mental and the neural–an idea that is supported by what is called 'methodological naturalism' which encourages applying the same scientific methodology to the study of both the mind and the body including the brain.
Sanz (2013) has pointed out that 'jump' as a verb can occur in a causative form in garden-path sentences, even though some native speakers of English may not grasp this immediately.
Here the term 'linguistic principles' indicates that the principles concerned are part and parcel of the psychological procedures and/or routines uniquely employed for (the processing of) linguistic structures, as also clarified in footnote 1.
In example (1), for instance, ‘the soldier’ (A) can be the set of soldiers containing a singleton entity in this case, ‘jumped near the barbed wire fences’ (B) is the set of those who jumped near the barbed wire fences and ‘finally collapsed’ is the set of those who finally collapsed. Thus, we have that A ⊆ B & A ⊆ C -->A⋂B ⊆ C where the linear sequence is A < B < C and ‘-->’ means ‘then it follows that’. This formulation can apply to (2–3) as well. In contrast, the interpretation of (4) turns on whether or not the-students⋂come-to-see-me-every-day holds, or the-professors ⋂come-to-see-me-every-day does. In (5) the straightforward interpretation is that of countries-in-the-world⋂do-not-execute-criminals. The bold part in each case for (4–5) is a set.
In order to better understand this point, one could think of a case where one thing is unequivocally instantiated in another. Suppose there is computer game or a simulation of evolutionary dynamics. Whatever happens when the software runs is not fully defined by the computer’s hardware, and there must be certain regularities defined at some other level (in this case, the software) such that even if we knew precisely what the computer hardware is and what it is capable of, we would still not fully know what the game or the simulation does. We would, however, know about some constraints that underlie what the game might do. But this does not give us a purchase on understanding why the regularities and patterns in the software are the way they are. Even a clear-cut case of instantiation does present this problem, and if so, we wonder why the system of patterns in linguistic cognition cannot when its relation to neurobiology is not clear enough.
The role of genetic variation/determination may seem relevant here because one may insist that components of language are ultimately coded and expressed by genetic networks. Hence the claim could be that the determination of cognitive properties of language can be traced to these genetic networks and their emergent expressions.
Even a full understanding of the neural implementation of language is unlikely to be sufficient to derive all the constraints and regularities that appear in language. No detailed account of the neuronal processes can account for why passive alternations, for example, are the way they are.
Here the concept of governing by mechanisms of genetic coding has to be understood as the preparation of the language-ready brain for the emergence of the language capacity as a whole which can be identified with an aspect (or dimension) of language distinct from the conception of language as consisting of internally complex parts such as syntax, morphology, phonology and semantics. This is discussed in detail in "Language-Biology Relations: A Revisionary View" section.
One plausible way of accounting for the mirroring patterns in the form of linguistic cognition shaped by the alternations in syntactic structures is to adopt the notion of synfire chains which encode serial structures of symbols within words and neuronal sequence detectors which can encode sequences of symbols such as words by means Hebbian learning of strengthening and weaning of connections (Pulvermüller 2002). Both synfire chains and neuronal sequence detectors are composed of neuronal webs that are a kind of highly connected neuronal assemblies. Further, this can also be achieved by means of certain oscillatory mechanisms through the binding of alpha and gamma rhythms in the brain that are sensitive to serial order (Murphy 2015). Regardless of whether or not neuronal chains or the binding of neural oscillatory rhythms does in reality capture the linguistic mirror reversal relation at hand, what is noteworthy is that these accounts re-describe the given linguistic relations in terms that are neither causal nor explanatorily adequate. Thus, for example, neuronal chains encoding order are actually chains encoding order with the term 'neuronal' adding just the substrate to the description. The description at hand in terms of chains encoding the serial order of symbols may also be advanced without any commitment to neuronal grounding, and if so, the description does not have to be, or is simply not, necessarily neuronal. Likewise, the binding of rhythms in the brain should not be unique to linguistic relations; nor are they explanatorily involved in certain selective linguistic relations but not others which are part of the logical complexity of language because the logical complexity of language, however dynamic it may be, is outside a strict spatio-temporal frame of reference whereas brain oscillations are, after all, dynamic processes occurring in real time (Buzsáki and Freeman 2015). Even though the logical complexity of language may change over time, its stabilized form (say, the distinction between sentence negation and constituent negation) at any time point is always outside the spatio-temporal frame of reference of brain oscillations (language as a phenomenon is at issue here, but not how people use language). Even though low-frequency brain rhythms (such as the delta oscillations) can have the coupling with the high-frequency brain rhythms (such as theta, beta, gama oscillations) to support the phrase structuring of linguistic strings in syntax (Murphy 2020), this does not establish that the logical properties of phrasal constituents (such as constituent negation, constituent coordination etc.) reside in the coupling. They are, after all, emergent properties that ride on brain-culture co-development. The answer concerned cannot be found in brain rhythms, crucially because bottom-up and top-down oscillatory processes run across cortical levels to maintain coherence in experiences of everything around us, not just language.
The discussion of two relevant dimensions of language here does not in any way presuppose that these are the only dimensions of language. In fact, there is also a view of language on which language is an array of embodied collective interpersonal activities that often serve social coordinating functions (see Thibault 2004). This view is also shared by most sociolinguists. Its importance for the present context is that this does not deny the role of biology in shaping interpersonal activities in language (Melrose 2005; see also Mondal 2012). Taken in this sense, this view in fact accords well with the notion of language as a linguistic capacity embodied and grounded in the socio-cultural niche humans live in. But this does not, of course, mean that the actual structuring of the collective interpersonal activities is itself to be found in biology.
Even though languages vary widely, certain syntactic patterns or rules do not occur in human language grammars, which suggests that there are biological constraints on human language grammars (see Tettamanti et al., 2002). This may indeed be the case, but the connection will not be direct. As argued here, neurobiological developmental constraints certainly can shape abstract properties of human language grammars indirectly via their control on the growth and gradual expression of the language capacity in development. One example can clarify this. For instance, the fact that no natural language grammar has a question formation rule that permutes the sequence of words in the inverse direction (say, < a, b, c, d, e > to < e, d, c, b, a >) has to do to sensory-motor and working-memory mechanisms, neural pathways for information integration, and mechanisms of sequential processing and motor programming/planning which are directly governed by neurobiological constraints.
Moro et al. (2001) has shown that syntactic processing can be isolated from lexical-semantic processing, especially in connection with the detection of anomalies in sentences consisting of pseudowords. This may suggest that the syntactic capacity can be actually isolated from the linguistic capacity as a whole. But this argument is fallacious, precisely because sentences consisting of pseudowords make sense only with reference to the linguistic capacity as a whole. It is precisely due to the possession of the linguistic capacity that we are able to detect patterns in sentences composed of pseudowords, for strings of pseudowords must conform to regular patterns found in natural language. Languageless creatures cannot make the distinction between human language words and possible human language pseudowords and then between human language sentences composed of words and sentences consisting of pseudowords (see Terrace 2019). Besides, processing sentences consisting of pseudowords may be a quite different thing from processing actual sentences in human language (see Pylkkänen 2019). Moreover, the experimental study of Röder et al. (2002) contradicts the findings of Moro et al. (2001), in that the effects of scrambling (word order alterations) for sentences with real words are found to be more pronounced in the left inferior frontal gyrus (especially, the Broca’s region) than for sentences containing pseudowords. If Broca’s region is exclusively for syntactic processing and not for semantic integration, then these syntactic processes for sentences containing pseudowords should be equally pronounced. But this was not observed. There is also no compelling neurobiological evidence till now that confirms that Broca’s region or any other brain region is solely dedicated to syntactic processing (Kaan and Swaab 2002; Rogalsky and Hickok 2011). In addition, contrary to the findings of Moro et al. (2001), the role of basal ganglia is not essential in morpho-syntactic processing (Longworth et al., 2005).
References
Anderson, J. R. (2007). How can the Human Mind Occur in the Physical Universe? Oxford University Press.
Anderson, M. L. (2014). After Phrenology: Neural Reuse and the Interactive Brain. MIT Press.
Armstrong, D. M. (1993). A Materialist Theory of the Mind. Routledge.
Bechtel, W. (2008). Mental Mechanisms: Philosophical Perspectives on Cognitive Neuroscience. Routledge.
Bechtel, W., & Graham, G. (1998). A Companion to Cognitive Science. Blackwell.
Ben Shalom, D., & Poeppel, D. (2008). Functional anatomic model of language: Assembling the pieces. The Neuroscientist, 1, 119–127.
Berwick, R., Friederici, A. D., Chomsky, N., & Bolhuis, J. J. (2013). Evolution, brain, and the nature of language. Trends in Cognitive Sciences, 17(2), 89–98.
Bickerton, D. (2014). Some problems for biolinguistics. Biolinguistics, 8, 73–96.
Boeckx, C. (2015). Beyond Humboldt’s problem: Reflections on biolinguistics and its relation to generative grammar. Language Sciences, 50, 127–132.
Boeckx, C. (2017). A conjecture about the neural basis of recursion in light of descent with modification. Journal of Neurolinguistics, 43(Part B), 193–198.
Bookheimer, S. (2002). Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annual Review Neuroscience, 25, 151–188.
Botha, R. (2016). Language Evolution: The Windows Approach. Cambridge University Press.
Brown, A. M. (1990). Development of visual sensitivity to light and color vision in human infants: a critical review. Vision Research, 30(8), 1159–1188.
Buzsáki, G. (2019). The Brain from Inside Out. Oxford University Press.
Buzsáki, G., & Freeman, W. (2015). Brain rhythms and dynamic coordination. Current Opinion in Neurobiology, 31, v–ix.
Cahana-Amitay, D. and Albert, M. L. (2014). Brain and language: evidence for neural multifunctionality. Behavioural Neurology 260381: https://doi.org/10.1155/2014/260381
Chater, N., Reali, F., & Christiansen, M. H. (2009). Restrictions on biological adaptation in language evolution. Proceedings of the National Academy of Sciences of the United States of America, 106(4), 1015–1020.
Chater, et al. (2015). Empiricism and Language Learnability. Oxford University Press.
Chomsky, N. (1995). The Minimalist Program. MIT Press.
Chomsky, N. (2000). New Horizons in the Study of Language and Mind. MIT Press.
Christiansen, M. H., & Chater, N. (2008). Language as shaped by the brain. Behavioral and Brain Sciences, 31, 489–558.
Christiansen, M. H., & Chater, N. (2016). Creating Language: Integrating Evolution, Acquisition, and Processing. MIT Press.
Churchland, P. M. (1992). A Neurocomputational Perspective: The Nature of Mind and the Structure of Science. MIT Press.
Civelek, M., & Lusis, A. J. (2014). Systems genetics approaches to understand complex traits. Nature Reviews Genetics, 15(1), 34–48.
Clifton, C. (2000). Evaluating models of human sentence processing. In M. W. Crocker, M. Pickering, & C. Clifton (Eds.), Architectures and Mechanisms for Language Processing (pp. 31–55). Cambridge University Press.
Craver, C. F. (2007). Explaining the Brain: Mechanisms and the Mosaic Unity of Neuroscience. Oxford University Press.
Crick, F. (1994). The Astonishing Hypothesis: The Scientific Search for the Soul. Charles Scribner’s Sons.
De Clercq, K. (2020). Types of negation. In V. Déprez & M. T. Espinal (Eds.), The Oxford Handbook of Negation (pp. 58–74). Oxford University Press.
Di Sciullo, A. M., & Boeckx, C. (Eds.). (2011). The Biolinguistic Enterprise: New Perspectives on the Evolution and Nature of the Human Language Faculty. Oxford University Press.
Dick, A. S., & Tremblay, P. (2012). Beyond the arcuate fasciculus: Consensus and controversy in the connectional anatomy of language. Brain, 135(12), 3529–3550.
Egan, F. (2017). Function-theoretic explanation and the search for neural mechanisms. In D. M. Caplan (Ed.), Explanation and Integration in Mind and Brain Science (pp. 145–163). Oxford University Press.
Eronen, M. I. (2015). Levels of organization: A deflationary account. Biology and Philosophy, 30, 39–58.
Embick, D., & Poeppel, D. (2014). Towards a computational(ist) neurobiology of language: Correlational, integrated and explanatory neurolinguistics. Language, Cognition and Neuroscience, 30(4), 357–366.
Fisher, S. E. (2006). Tangled webs: Tracing the connections between genes and cognition. Cognition, 101(2), 270–297.
Fisher, S. E. (2017). Evolution of language: Lessons from the genome. Psychonomic Bulletin and Review, 24(1), 34–40.
Frank, M. C., Vul, E., & Johnson, S. P. (2009). Development of infants’ attention to faces during the first year. Cognition, 110(2), 160–170.
Frazier, L., & Clifton, C. (1996). Construal. MIT Press.
Friederici, A. D. (2011). The brain basis of language processing: From structure to function. Physiological Reviews, 91(4), 1357–1392.
Friederici, A. D. (2017a). Neurobiology of syntax as the core of human language. Biolinguistics, 11, 325–337.
Friederici, A. D. (2017b). Language in Our Brain: The Origins of a Uniquely Human Capacity. MIT Press.
Friederici, A. D., Bahlmann, J., Friedrich, R., & Makuuchi, M. (2011). The neural basis of recursion and complex syntactic hierarchy. Biolinguistics, 5(1–2), 87–104.
Garnham, A. (2005). Language comprehension. In K. Lamberts & R. L. Goldstone (Eds.), Handbook of Cognition (pp. 1–5). Sage Publications.
Geschwind, N. (1972). The organization of language and the brain. Science, 170, 940–944.
Gibson, J. J. (1979). The Ecological Approach to Visual Perception. Houghton Mifflin.
Godfrey-Smith, P. (2007). Innateness and genetic information. Carruthers, P., Laurence, S. and Stich, S. (Eds.). The Innate Mind: Foundations and the Future, Vol. 3, 55–68. Oxford University Press.
Grimaldi, M. (2012). Toward a neural theory of language: Old issues and new perspectives. Journal of Neurolinguistics, 25(5), 304–327.
Hagoort, P. (2014). Nodes and networks in the neural architecture for language: Broca’s area and beyond. Current Opinion in Neurobiology, 28, 136–141.
Hagoort, P. (2019). The neurobiology of language beyond single-word processing. Science, 366(6461), 55–58.
Hagoort, P., & Beckman, C. F. (2019). Key issues and future directions: The neural architecture for language. In P. Hagoort (Ed.), Human Language: From Genes and Brains to Behavior (pp. 527–532). MIT Press.
Haugeland, J. (1978). The nature and plausibility of cognitivism. Behavioral and Brain Sciences, 1, 215–226.
Hauser, M. D., & Watumull, J. (2017). The Universal Generative Faculty: The source of our expressive power in language, mathematics, morality, and music. Journal of Neurolinguistics, 43, 78–94.
Hauser, M. D., Chomsky, N., & Fitch, T. (2002). The faculty of language: What is it, who has it, and how did it evolve? Science, 298, 1569–1579.
Hickok, G. (2019). Functional anatomy of speech production: From words to motor control. In P. Hagoort (Ed.), Human Language: From Genes and Brains to Behavior (pp. 381–392). MIT Press.
Hillert, D. G. (2015). On the evolving biology of language. Frontiers in Psychology, 6, 1796.
Horst, S. (2007). Beyond Reduction: Philosophy of Mind and Post-Reductionist Philosophy of Science. Oxford University Press.
Indefrey, P., & Levelt, W. J. (2004). The spatial and temporal signatures of word production components. Cognition, 92, 101–144.
Jablonka, E., & Ginsburg, S. (2019). The Evolution of the Sensitive Soul. MIT Press.
Jackendoff, R. (1997). The Architecture of the Language Faculty. MIT Press.
Jackendoff, R. (2002). Foundations of Language: Brain, Meaning, Grammar, Evolution. Oxford University Press.
Jakobson, R. (1956). Two aspects of language and two types of aphasic disturbances. In R. Jakobson & M. Halle (Eds.), Fundamentals of Language (pp. 115–133). Mouton de Gruyter.
Jenkins, L. (2001). Biolinguistics: Exploring the Biology of Language. Cambridge University Press.
Johnson, G. (2009). Mechanisms and functional brain areas. Mind and Machines, 19, 255–271.
Kaan, E., & Swaab, T. Y. (2002). The brain circuitry of syntactic comprehension. Trends in Cognitive Sciences, 6(8), 350–356.
Kemmerer, D. (2014). Cognitive Neuroscience of Language. Psychology Press.
Kim, J. (2011). Philosophy of Mind. Westview Press.
Laland, K. N. (2017). Darwin’s Unfinished Symphony: How Culture Made the Human Mind. Princeton University Press.
Langacker, R. (1987). Foundations of Cognitive Grammar. Stanford University Press.
Langacker, R. (1999). Grammar and Conceptualization. Mouton de Gruyter.
Langley, P., Laird, J. E., & Rogers, S. (2009). Cognitive architectures: Research issues and challenges. Cognitive Systems Research, 10(2), 141–160.
Lenneberg, E. H. (1967). Biological Foundations of Language. Wiley.
Lichtheim L. (1885). On aphasia. Brain, 7(4), 433–484.
Lieberman, P. (2000). Human Language and Our Reptilian Brain. Harvard University Press.
Lieberman, P. (2006). Towards an Evolutionary Biology of Language. Harvard University Press.
Lieberman, P. (2013). The Unpredictable Species. Princeton University Press.
Longworth, C. E., et al. (2005). The basal ganglia and rule-governed language use: Evidence from vascular and degenerative conditions. Brain, 128(3), 584–596.
Luria, S. E. (1973). Life, the Unfinished Experiment. Charles Scribner’s Sons.
MacWhinney, B. (2005). Language evolution and human development. In B. Ellis & D. Bjorklund (Eds.), Origins of the Social Mind (pp. 383–410). Guilford.
MacWhinney, B. (Ed.) (1999). The Emergence of Language. New York Psychology Press.
MacWhinney, B. (2002). The emergence of language from body, brain, and society. In Andronis, M., Debenport, E., Pycha, A. and Yoshimura, K. (Eds). Proceedings from the Panels of Thirty-Eighth Meeting of the Chicago Linguistic Society, 147–171. Vol. 38(2). Chicago: Chicago Linguistic Society.
Marr, D. (1982). Vision: A Computational Investigation into the Human Representation and Processing of Visual Information. W. H. Freeman.
Melrose, R. (2005). How a neurological account of language can be reconciled with a linguist’s account of language: The case of systemic-functional linguistics. Journal of Neurolinguistics, 18(5), 401–421.
Mitchell, D. C., Brysbaert, M., Grondelaers, S., & Swanepoel, P. (2000). Modifier attachment in Dutch: Testing aspects of Construal Theory. In A. Kennedy, R. Radach, D. Heller, & J. Pynte (Eds.), Reading as a Perceptual Process (pp. 493–516). Elsevier.
Mondal, P. (2012). Can internalism and externalism be reconciled in a biological epistemology of language? Biosemiotics, 5, 61–82.
Mondal, P. (2014). Language, Mind and Computation. Palgrave Macmillan.
Mondal, P. (2019). Language, Biology and Cognition: A Critical Perspective. Springer Nature.
Mondal, P. (2022a). Disunity with unity in cognition within the context of language–biology relations. Journal of Theoretical and Philosophical Psychology, 42(1), 19–36.
Mondal, P. (2022b). The puzzling chasm between cognitive representations and formal structures of linguistic meanings. Cognitive Science, 46(9), e13200.
Moro, A., et al. (2001). Syntax and the brain: Disentangling grammar by selective anomalies. NeuroImage, 13(1), 110–118.
Murphy, E. (2020). The Oscillatory Nature of Language. Cambridge University Press.
Murphy, E. (2015). The brain dynamics of linguistics computation. In Boeckx, C. and Benitez-Burraco, A. (Eds.). Components of the Language-ready Brain, 50–68. Ebook: Frontiers in Psychology.
Newell, A. (1994). Unified Theories of Cognition. Cambridge, Mass.: Harvard University Press.
Northoff, G. (2018). The Spontaneous Brain: From Mind-Body Problem to World-Brain Problem. MIT Press.
Poeppel, D. (2014). The neuroanatomic and neurophysiological infrastructure for speech and language. Current Opinion in Neurobiology, 28, 142–149.
Poeppel, D., & Embick, D. (2005). Defining the Relation between Linguistics and Neuroscience. In A. Cutler (Ed.), Twenty-First Century Psycholinguistics: Four Cornerstones (pp. 103–118). Lawrence Erlbaum.
Poeppel, D., & Hickok, G. (2004). Towards a new functional anatomy of language. Cognition, 92(1–2), 1–12.
Poeppel, D. (2008). The cartographic imperative: confusing localization and explanation in human brain mapping. In Bre-dekamp, H., Werner, G. and Bruhn, M. (Eds.). Bildwelten des Wissens: Ikonographie des Gehirns, 19–29, (Bildwelten des Wissen; Vol. 6.1).
Postal, P. (2009). The incoherence of Chomsky’s “biolinguistic” ontology. Biolinguistics, 3(1), 104–123.
Pulvermüller, F. (2002). The Neuroscience of Language: On Brain Circuits of Words and Serial Order. Cambridge University Press.
Putnam, H. (1967). Psychological predicates. In W. H. Capitan & D. D. Merrill (Eds.), Art, Mind, and Religion (pp. 37–48). University of Pittsburgh Press.
Pylkkänen, L. (2019). The neural basis of combinatory syntax and semantics. Science, 366(6461), 62–66.
Röder, et al. (2002). Brain activation modulated by the comprehension of normal and pseudo-word sentences of different processing demands: A functional magnetic resonance imaging study. NeuroImage, 15(4), 1003–1014.
Rogalsky, C., & Hickok, G. (2011). The role of Broca’s area in sentence comprehension. Journal of Cognitive Neuroscience, 23(7), 1664–1680.
Rolls, E. T. (2021). Mind causality: A computational neuroscience approach. Frontiers in Computational Neuroscience, 15, 706505. https://doi.org/10.3389/fncom.2021.706505
Sanz, M. (1996). Telicity, objects and the mapping onto predicate types: A cross-linguistic study of the role of syntax in processing. Doctoral Dissertation: University of Rochester.
Sanz, M. (2013). The path from certain events to linguistic uncertainties. Sanz, M., Laka, I. and Tanenhaus, M. K. (Eds.). Language down the Garden Path: The Cognitive and Biological Basis for Linguistic Structures, 253–262. New York: Oxford University Press.
Smolensky, P., & Legendre, G. (2006). The Harmonic Mind. MIT Press.
Sokol, S. (1978). Measurement of infant visual acuity from pattern reversal evoked potentials. Vision Research, 18(1), 33–39.
Sperry, R. W. (1980). Mind-brain interaction: Mentalism, yes; dualism, no. Neuroscience, 5(2), 195–206.
Stromswold, K. (2008). The genetics of speech and language disorders. New England Journal of Medicine, 359(22), 2381–2383.
Sun, L., & Wu, R. (2015). Mapping complex traits as a dynamic system. Physics of Life Reviews, 13, 155–185.
Talmy, L. (2000). Towards a Cognitive Semantics. 2 vols. MIT Press.
Terrace, H. S. (2019). Why Chimpanzees Can’t Learn Language and Only Humans Can. Columbia University Press.
Tettamanti, M., et al. (2002). Neural correlates for the acquisition of natural language syntax. NeuroImage, 17(2), 700–709.
Thibault, P. J. (2004). Brain, Mind, and the Signifying Body: An Ecosocial Semiotic Theory. Continuum.
Townsend, D. J., & Bever, T. G. (2001). Sentence Comprehension: The Integration of Habits and Rules. MIT Press.
Tremblay, P., & Dick, A. S. (2016). Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain and Language, 162, 60–71.
Tsuruhara, A., et al. (2014). Infants’ ability to respond to depth from the retinal size of human faces: Comparing monocular and binocular preferential-looking. Infant Behavior & Development, 37(4), 562–570.
West-Eberhard, J. (2003). Developmental Plasticity and Evolution. Oxford University Press.
Westerlund, M., & Pylkkänen, L. (2014). The role of the left anterior temporal lobe in semantic composition vs. semantic memory. Neuropsychologia, 57, 59–70.
Zhang, L., & Pylkkänen, L. (2015). The interplay of composition and concept specificity in the left anterior temporal lobe: An MEG study. NeuroImage, 111, 228–240.
Zlatev, J., & Blomberg, J. (2015). Language may indeed influence thought. Frontiers in Psychology, 6, 1631. https://doi.org/10.3389/fpsyg.2015.01631
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
The author declares no known conflicts of interest/competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mondal, P. A Critical Perspective on the (Neuro)biological Foundations of Language and Linguistic Cognition. Integr. psych. behav. (2022). https://doi.org/10.1007/s12124-022-09741-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s12124-022-09741-0