Abstract
Background
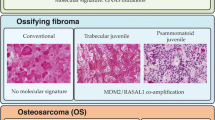
Ossifying fibroma (OF) of the craniofacial skeleton is a fibro-osseous lesion characterized by various patterns of bone formation in a cellular fibroblastic stroma. The molecular landscape of OF remains mostly unknown. There are a few known pathogenic abnormalities in OF, including HRPT2 mutations in conventional OF and SATB2 translocations in juvenile psammomatoid OF. On the other hand, conflicting reports exist regarding MDM2 gene amplification and chromosomal copy number alterations (CNA) in OF.
Methods
Surgically removed biopsies and curettage specimens from OF patients were obtained. Clinical, radiographic, and pathologic features of tumors were reviewed. Genomic DNA was extracted from formalin-fixed, paraffin-embedded blocks of tumor tissue. Capture-based DNA next-generation sequencing targeting the coding regions 529 cancer genes and select introns was performed.
Results
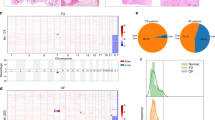
We identified 17 OF cases from 8 male and 8 female patients with mean age of 22 years (range 1–58 years). Nine case occurred in the gnathic bones and 8 in the extragnathic craniofacial bones. These cases included 3 juvenile psammomatoid OF, 6 conventional OF and 8 juvenile trabecular OF. Large-scale CNAs were present in 6 of 17 cases. Seven cases (41%) had focal amplifications including FOSB (n = 2, 11%), FOS (n = 4, 23%), COL1A1 (n = 4, 23%) and TBX3 (n = 5, 29%). Three cases (17%) had pathogenic CDC73 mutations. No cases showed focal MDM2 amplification.
Conclusions
Here, we provided a comprehensive molecular characterization of OF that reveals a heterogeneous genetic profile with occasional large-scale CNAs (n = 6, 35%). FOS, FOSB, and TBX3 genes that regulate AP-1 transcriptional complex are frequently altered in OF (n = 7, 41%), chiefly in juvenile trabecular OF. These genes encode transcription factors that act as downstream effectors of the MAP kinase signaling pathway. MDM2 amplification is an exceedingly rare event in OF, if present at all, so identification of this event should continue to raise concern for low-grade gnathic osteosarcoma. In summary, our findings suggest that OF represents a heterogeneous group of tumors at the genetic level but dysregulation of the AP-1 pathway may play a role in pathogenesis of juvenile trabecular OF.



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Disclosures
Data from this manuscript was presented, in part, at the annual meeting of United States and Canadian Academy of Pathology, with an embargo on publication until its completion, March 16, 2023.
The full research will be published in Head and Neck Pathology.
References
El Mofty SK, Nelson B, Toyosawa S (2017) Fibro-osseous and osteochondromatous lesions. In: WHO Classification of Head and Neck Tumors. IARC Press, Lyon. pp 251–255.
Hameed M, Horvai AE, Jordan RCK (2020) Soft tissue special issue: gnathic fibro-osseous lesions and osteosarcoma. Head Neck Pathol 14(1):70–82
Shi RR, Li XF, Zhang R, Chen Y, Li TJ (2013) GNAS mutational analysis in differentiating fibrous dysplasia and ossifying fibroma of the jaw. Mod Pathol 26(8):1023–1031
Tabareau-Delalande F, Collin C, Gomez-Brouchet A et al (2013) Diagnostic value of investigating GNAS mutations in fibro-osseous lesions: a retrospective study of 91 cases of fibrous dysplasia and 40 other fibro-osseous lesions. Mod Pathol 26(7):911–921
Jour G, Oultache A, Sadowska J et al (2016) GNAS mutations in fibrous dysplasia: a comparative study of standard sequencing and locked nucleic acid PCR sequencing on decalcified and nondecalcified formalin-fixed paraffin-embedded tissues. Appl Immunohistochem Mol Morphol 24(9):660–667
Dujardin F, Binh MB, Bouvier C et al (2011) MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol 24(5):624–637
Limbach AL, Lingen MW, McElherne J et al (2020) The utility of MDM2 and CDK4 immunohistochemistry and MDM2 FISH in craniofacial osteosarcoma. Head Neck Pathol 14(4):889–898
Newey PJ, Bowl MR, Cranston T, Thakker RV (2010) Cell division cycle protein 73 homolog (CDC73) mutations in the hyperparathyroidism-jaw tumor syndrome (HPT-JT) and parathyroid tumors. Hum Mutat 31(3):295–307
Pereira TDSF, Diniz MG, França JA et al (2018) The Wnt/β-catenin pathway is deregulated in cemento-ossifying fibromas. Oral Surg Oral Med Oral Pathol Oral Radiol 125(2):172–178
Horvai AE, Jordan RC (2014) Fibro-osseous lesions of the craniofacial bones: β-catenin immunohistochemical analysis and CTNNB1 and APC mutation analysis [published correction appears in Head Neck Pathol. 2014 Sep;8(3):369]. Head Neck Pathol 8(3):291–297
Tabareau-Delalande F, Collin C, Gomez-Brouchet A et al (2015) Chromosome 12 long arm rearrangement covering MDM2 and RASAL1 is associated with aggressive craniofacial juvenile ossifying fibroma and extracranial psammomatoid fibro-osseous lesions. Mod Pathol 28(1):48–56
Ma M, Liu L, Shi R et al (2021) Copy number alteration profiling facilitates differential diagnosis between ossifying fibroma and fibrous dysplasia of the jaws. Int J Oral Sci 13(1):21
Bahceci DH, Jordan RCK, Horvai AE (2022) MDM2 gene amplification and expression of MDM2 and CDK4 are rare in ossifying fibroma of craniofacial bones. Head Neck Pathol. https://doi.org/10.1007/s12105-022-01454-5
Sawyer JR, Tryka AF, Bell JM, Boop FA (1995) Nonrandom chromosome breakpoints at Xq26 and 2q33 characterize cemento-ossifying fibromas of the orbit. Cancer 76(10):1853–1859
Cleven A, Szuhai K, van IJzendoorn D et al (2022) Juvenile psammomatoid ossifying fibroma is defined by SATB2 rearrangement. Mod Pathol 35(Suppl 2):866
Baumhoer D, Haefliger S, Ameline B et al (2022) Ossifying fibroma of non-odontogenic origin: a fibro-osseous lesion in the craniofacial skeleton to be (re-)considered. Head Neck Pathol 16(1):257–267
Milde-Langosch K (2005) The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer 41(16):2449–2461
Whitmarsh AJ, Davis RJ (1996) Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med (Berl) 74(10):589–607
Eferl R, Wagner EF (2003) AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3(11):859–868
Wagner EF (2002) Functions of AP1 (Fos/Jun) in bone development. Ann Rheum Dis 61(Suppl 2):ii40–ii42.
Bozec A, Bakiri L, Jimenez M, Schinke T, Amling M, Wagner EF (2010) Fra-2/AP-1 controls bone formation by regulating osteoblast differentiation and collagen production. J Cell Biol 190(6):1093–1106
Grigoriadis AE, Wang ZQ, Wagner EF (1995) Fos and bone cell development: lessons from a nuclear oncogene. Trends Genet 11(11):436–441
Fleischmann A, Hafezi F, Elliott C, Remé CE, Rüther U, Wagner EF (2000) Fra-1 replaces c-Fos-dependent functions in mice. Genes Dev 14(21):2695–2700
Takayanagi H, Kim S, Matsuo K et al (2002) RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature 416(6882):744–749
Lewinson D, Rachmiel A, Rihani-Bisharat S et al (2003) Stimulation of Fos- and Jun-related genes during distraction osteogenesis. J Histochem Cytochem 51(9):1161–1168
Miller AD, Curran T, Verma IM (1984) C-Fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell 36(1):51–60
van IJzendoorn DG, de Jong D, Romagosa C et al (2015) Fusion events lead to truncation of FOS in epithelioid hemangioma of bone. Genes Chromosomes Cancer 54(9):565–574
Huang SC, Zhang L, Sung YS et al (2015) Frequent FOS gene rearrangements in epithelioid hemangioma: a molecular study of 58 cases with morphologic reappraisal. Am J Surg Pathol 39(10):1313–1321
Walther C, Tayebwa J, Lilljebjörn H et al (2014) A novel SERPINE1-FOSB fusion gene results in transcriptional up-regulation of FOSB in pseudomyogenic haemangioendothelioma. J Pathol 232(5):534–540
Antonescu CR, Chen HW, Zhang L et al (2014) ZFP36-FOSB fusion defines a subset of epithelioid hemangioma with atypical features. Genes Chromosomes Cancer 53(11):951–959
Fittall MW, Mifsud W, Pillay N et al (2018) Recurrent rearrangements of FOS and FOSB define osteoblastoma. Nat Commun 9(1):2150
Amary F, Markert E, Berisha F et al (2019) FOS expression in osteoid osteoma and osteoblastoma: a valuable ancillary diagnostic tool. Am J Surg Pathol 43(12):1661–1667
Lam SW, Cleven AHG, Briaire-de Bruijn IH et al (2021) FOS rearrangement and expression in cementoblastoma. Am J Surg Pathol 45(5):690–693
van IJzendoorn DGP, Forghany Z, Liebelt F et al (2017) Functional analyses of a human vascular tumor FOS variant identify a novel degradation mechanism and a link to tumorigenesis. J Biol Chem 292(52):21282–21290
van IJzendoorn DGP, Bovée JVMG (2017) Vascular tumors of bone: the evolvement of a classification based on molecular developments. Surg Pathol Clin 10(3):621–635
Candeliere GA, Glorieux FH, Prud’homme J, St-Arnaud R (1995) Increased expression of the c-Fos proto-oncogene in bone from patients with fibrous dysplasia. N Engl J Med 332(23):1546–1551
Ghosh TK, Packham EA, Bonser AJ, Robinson TE, Cross SJ, Brook JD (2001) Characterization of the TBX5 binding site and analysis of mutations that cause Holt-Oram syndrome. Hum Mol Genet 10(18):1983–1994
Carlson H, Ota S, Campbell CE, Hurlin PJ (2001) A dominant repression domain in Tbx3 mediates transcriptional repression and cell immortalization: relevance to mutations in Tbx3 that cause ulnar-mammary syndrome. Hum Mol Genet 10(21):2403–2413
Washkowitz AJ, Gavrilov S, Begum S, Papaioannou VE (2012) Diverse functional networks of Tbx3 in development and disease. Wiley Interdiscip Rev Syst Biol Med 4(3):273–283
Khan SF, Damerell V, Omar R et al (2020) The roles and regulation of TBX3 in development and disease. Gene 726:144223
Coll M, Seidman JG, Müller CW (2002) Structure of the DNA-bound T-box domain of human TBX3, a transcription factor responsible for ulnar-mammary syndrome. Structure 10(3):343–356
Wansleben S, Peres J, Hare S, Goding CR, Prince S (2014) T-box transcription factors in cancer biology. Biochim Biophys Acta 1846(2):380–391
Mowla S, Pinnock R, Leaner VD, Goding CR, Prince S (2011) PMA-induced up-regulation of TBX3 is mediated by AP-1 and contributes to breast cancer cell migration. Biochem J 433(1):145–153
Chen JD, Morrison C, Zhang C, Kahnoski K, Carpten JD, Teh BT (2003) Hyperparathyroidism-jaw tumour syndrome. J Intern Med 253(6):634–642
Carpten JD, Robbins CM, Villablanca A et al (2002) HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet 32(4):676–680
Costa-Guda J, Pandya C, Strahl M et al (2021) Parafibromin abnormalities in ossifying fibroma. J Endocr Soc 5(7):bvab087
Chen Y, Hu DY, Wang TT et al (2016) CDC73 gene mutations in sporadic ossifying fibroma of the jaws. Diagn Pathol 11(1):91
Raymond VM, Gray SW, Roychowdhury S et al (2015) Germline findings in tumor-only sequencing: points to consider for clinicians and laboratories. J Natl Cancer Inst 108(4):djv351
Funding
The study was funded by the University California at San Francisco Department of Pathology, Clinical Research Endowment.
Author information
Authors and Affiliations
Contributions
All authors confirm they have meaningfully contributed to the research and read and approved the final manuscript. Data from this manuscript was presented, in part, at the annual meeting of United States and Canadian Academy of Pathology, with an embargo on publication until its completion, March 16, 2023.The full research will be published in Head and Neck Pathology.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study has obtained IRB approval the Institutional Review Board at the University of California, San Francisco (IRB#11-05361). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Participate
The need for informed consent was waived by the Institutional Review Board at the University of California, San Francisco (IRB#11-05361).
Consent for Publication
This type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bahceci, D.H., Grenert, J.P., Jordan, R.C.K. et al. Genomic Profiling of the Craniofacial Ossifying Fibroma by Next-Generation Sequencing. Head and Neck Pathol 17, 722–730 (2023). https://doi.org/10.1007/s12105-022-01523-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-022-01523-9