Abstract
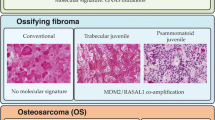
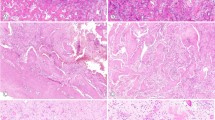
Gnathic fibro-osseous lesions are a diverse group of disease processes which share overlapping microscopic features characterized by fibroblastic stroma with variable cellularity and a range of bone forming pathological processes leading to woven, sclerotic and cementum-like structures. Some of the lesions are unique to craniofacial location and a combination of clinical, radiological and pathological correlation is often necessary for diagnostic accuracy. Gnathic osteosarcomas are rare tumors with differences in age distribution and behavior as compared to osteosarcoma of long bones. This review will discuss the clinicopathological and radiological features of gnathic fibro-osseous lesions and osteosarcoma with updates on current genetics and molecular pathogenesis.








Similar content being viewed by others
References
Regezi J, Sciubba J, Jordan R. Odontogenic tumors. Oral pathology: clinical pathology correlates. 6th ed. Amsterdam: Elsevier; 2012. p. 285–7.
Eversole R, Su L, ElMofty S. Benign fibro-osseous lesions of the craniofacial complex. A review. Head Neck Pathol. 2008;2:177–202.
Kuratani S. Evolution of the vertebrate jaw: comparative embryology and molecular developmental biology reveal the factors behind evolutionary novelty. J Anat. 2004;205:335–47.
Koury ME, Regezi JA, Perrott DH, Kaban LB. “Atypical” fibro-osseous lesions: diagnostic challenges and treatment concepts. Int J Oral Maxillofac Surg. 1995;24:162–9.
El Mofty SK, Nelson B, Toyosawa S. Fibro-osseous and osteochondromatous lesions. In: WHO Classification of Head and Neck Tumors. Lyon: IARC Press; 2017. pp. 251–255.
Eversole LR, Leider AS, Nelson K. Ossifying fibroma: a clinicopathologic study of sixty-four cases. Oral Surg Oral Med Oral Pathol. 1985;60:505–11.
Regezi J, Sciubba J, Jordan R. Benign non-odontogenic tumors. In: Regezi J, Sciubba J, Jordan R, editors. Oral pathology: clinical pathology correlates. 6th ed. Amsterdam: Elsevier; 2012. p. 294–8.
Carpten JD, Robbins CM, Villablanca A, et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet. 2002;32:676–80.
Pimenta FJ, Gontijo Silveira LF, Tavares GC, et al. HRPT2 gene alterations in ossifying fibroma of the jaws. Oral Oncol. 2006;42:735–9.
de Mesquita Netto AC, Gomez RS, Diniz MG, Fonseca-Silva T, Campos K, De Marco L, Carlos R, Gomes CC. Assessing the contribution of HRPT2 to the pathogenesis of jaw fibrous dysplasia, ossifying fibroma, and osteosarcoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:359–67.
Shi RR, Li XF, Zhang R, Chen Y, Li TJ. GNAS mutational analysis in differentiating fibrous dysplasia and ossifying fibroma of the jaw. Mod Pathol. 2013;26:1023–31.
Eh A, Cj R. Fibro-osseous lesions of the craniofacial bones: beta-catenin immunohistochemical analysis and CTNNB1 and APC mutation analysis. Head Neck Pathol. 2014;8:291–7.
Pereira T, Diniz MG, Franca JA, et al. The Wnt/beta-catenin pathway is deregulated in cemento-ossifying fibromas. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:172–8.
El-Mofty S. Psammomatoid and trabecular juvenile ossifying fibroma of the craniofacial skeleton: two distinct clinicopathologic entities. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:296–304.
Slootweg PJ, Muller H. Juvenile ossifying fibroma. Report of four cases. J Craniomaxillofac Surg. 1990;18:125–9.
Slootweg PJ, Panders AK, Koopmans R, Nikkels PG. Juvenile ossifying fibroma. An analysis of 33 cases with emphasis on histopathological aspects. J Oral Pathol Med. 1994;23:385–8.
Chrcanovic BR, Gomez RS. Juvenile ossifying fibroma of the jaws and paranasal sinuses: a systematic review of the cases reported in the literature. Int J Oral Maxillofac Surg. 2019. https://doi.org/10.1016/j.ijom.2019.06.029.
Wang K, Ma XJ, Hao SY, Du J, Zhang LW, Zhang JT, Wu Z. Skull base juvenile psammomatoid ossifying fibroma: clinical characteristics, treatment, and prognosis. World Neurosurg. 2019;125:e843–8.
Hasselblatt M, Jundt G, Greiner C, Rama B, Schmal F, Iglesias-Rozas JR, van de Nes JA, Paulus W. Juvenile psammomatoid ossifying fibroma of the neurocranium. Report of four cases. Journal of neurosurgery. 2005;102:1151–4.
Das BK, Das SN, Gupta A, Nayak S. Florid cemento-osseous dysplasia. J Oral Maxillofac Pathol. 2013;17:150.
Fenerty S, Shaw W, Verma R, Syed AB, Kuklani R, Yang J, Ali S. Florid cemento-osseous dysplasia: review of an uncommon fibro-osseous lesion of the jaw with important clinical implications. Skeletal Radiol. 2017;46:581–90.
Mahomed F, Altini M, Meer S, Coleman H. Cemento-osseous dysplasia with associated simple bone cysts. J Oral Maxillofac Surg. 2005;63:1549–54.
Sedano HO, Kuba R, Gorlin RJ. Autosomal dominant cemental dysplasia. Oral Surg Oral Med Oral Pathol. 1982;54:642–6.
Coleman H, Altini M, Kieser J, Nissenbaum M. Familial florid cemento-osseous dysplasia–a case report and review of the literature. J Dent Assoc S Afr. 1996;51:766–70.
Kahn MF, Hayem F, Hayem G, Grossin M. Is diffuse sclerosing osteomyelitis of the mandible part of the synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome? Analysis of seven cases. Oral Surg Oral Med Oral Pathol. 1994;78:594–8.
Kodama Y, Tanaka R, Kurokawa A, Ohnuki H, Sultana S, Hayashi T, Iizuka T, Takagi R. Severe destruction of the temporomandibular joint with complete resorption of the condyle associated with synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:e128–33.
Regezi J, Scriubba J, Jordan R. Inflammatory jaw lesions. In: Regezi J, Scriubba J, Jordan R, editors. Oral pathology clinical pathology correlates. 6th ed. Amsterdam: Elsevier; 2012. p. 324–5.
Muller-Richter UD, Roldan JC, Mortl M, Behr M, Reichert TE, Driemel O. SAPHO syndrome with ankylosis of the temporomandibular joint. Int J Oral Maxillofac Surg. 2009;38:1335–41.
Byrd L, Grossmann M, Potter M, Shen-Ong GL. Chronic multifocal osteomyelitis, a new recessive mutation on chromosome 18 of the mouse. Genomics. 1991;11:794–8.
Abe K, Cox A, Takamatsu N, et al. Gain-of-function mutations in a member of the Src family kinases cause autoinflammatory bone disease in mice and humans. Proc Natl Acad Sci USA. 2019;116:11872–7.
Mari A, Morla A, Melero M, Schiavone R, Rodriguez J. Diffuse sclerosing osteomyelitis (DSO) of the mandible in SAPHO syndrome: a novel approach with anti-TNF therapy. Systematic review. J Craniomaxillofac Surg. 2014;42:1990–6.
Hallmer F, Korduner M, Moystad A, Bjornland T. Treatment of diffuse sclerosing osteomyelitis of the jaw with denosumab shows remarkable results—a report of two cases. Clin Case Rep. 2018;6:2434–7.
Chapurlat RD, Orcel P. Fibrous dysplasia of bone and McCune-Albright syndrome. Best Pract Res Clin Rheumatol. 2008;22:55–69.
Lucas R. Pathology of tumors of oral tissues. 5th ed. London: Churchill Livingstone; 1998.
Waldron CA. Fibro-osseous lesions of the jaws. J Oral Maxillofac Surg. 1993;51:828–35.
El-Mofty S. Bone lesions. In: Gnepp D, editor. Diagnostic surgical pathology of the head and neck. Philadelphia: Saunders, Elsevier; 2009.
Fitzpatrick KA, Taljanovic MS, Speer DP, Graham AR, Jacobson JA, Barnes GR, Hunter TB. Imaging findings of fibrous dysplasia with histopathologic and intraoperative correlation. AJR Am J Roentgenol. 2004;182:1389–98.
Clauser L, Marchetti C, Piccione M, Bertoni F. Craniofacial fibrous dysplasia and Ollier’s disease: combined transfrontal and transfacial resection using the nasal-cheek flap. J Craniofac Surg. 1996;7:140–4.
MacMahon H. Albright’s syndrome—thirty years later (polyostotic fibrous dysplasia). Pathol Annu. 1976;6:81–146.
Shenker A, Weinstein LS, Sweet DE, Spiegel AM. An activating Gs alpha mutation is present in fibrous dysplasia of bone in the McCune-Albright syndrome. J Clin Endocrinol Metab. 1994;79:750–5.
Happle R. The McCune-Albright syndrome: a lethal gene surviving by mosaicism. Clin Genet. 1986;29:321–4.
Tabareau-Delalande F, Collin C, Gomez-Brouchet A, et al. Diagnostic value of investigating GNAS mutations in fibro-osseous lesions: a retrospective study of 91 cases of fibrous dysplasia and 40 other fibro-osseous lesions. Mod Pathol. 2013;26:911–21.
Lee SE, Lee EH, Park H, et al. The diagnostic utility of the GNAS mutation in patients with fibrous dysplasia: meta-analysis of 168 sporadic cases. Hum Pathol. 2012;43:1234–42.
Lietman SA, Ding C, Levine MA. A highly sensitive polymerase chain reaction method detects activating mutations of the GNAS gene in peripheral blood cells in McCune-Albright syndrome or isolated fibrous dysplasia. J Bone Joint Surg Am Vol. 2005;87:2489–94.
Mariot V, Wu JY, Aydin C, Mantovani G, Mahon MJ, Linglart A, Bastepe M. Potent constitutive cyclic AMP-generating activity of XLalphas implicates this imprinted GNAS product in the pathogenesis of McCune-Albright syndrome and fibrous dysplasia of bone. Bone. 2011;48:312–20.
Jour G, Oultache A, Sadowska J, Mitchell T, Healey J, Nafa K, Hameed M. GNAS Mutations in Fibrous Dysplasia: a Comparative Study of Standard Sequencing and Locked Nucleic Acid PCR Sequencing on Decalcified and Nondecalcified Formalin-fixed Paraffin-embedded Tissues. Appl Immunohistochem Mol Morphol. 2016;24:660–7.
Lee JS, FitzGibbon EJ, Chen YR, Kim HJ, Lustig LR, Akintoye SO, Collins MT, Kaban LB. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis. 2012;7(Suppl 1):S2.
Ruggieri P, Sim FH, Bond JR, Unni KK. Malignancies in fibrous dysplasia. Cancer. 1994;73:1411–24.
Tanner HC Jr, Dahlin DC, Childs DS Jr. Sarcoma complicating fibrous dysplasia. Probable role of radiation therapy. Oral Surg Oral Med Oral Pathol. 1961;14:837–46.
Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–34.
Tran LM, Mark R, Meier R, Calcaterra TC, Parker RG. Sarcomas of the head and neck. Prognostic factors and treatment strategies. Cancer. 1992;70:169–77.
Fernandes R, Nikitakis NG, Pazoki A, Ord RA. Osteogenic sarcoma of the jaw: a 10-year experience. J Oral Maxillofac Surg. 2007;65:1286–91.
Sato M, Tanaka N, Sato T, Amagasa T. Oral and maxillofacial tumours in children: a review. Br J Oral Maxillofac Surg. 1997;35:92–5.
McHugh JB, Thomas DG, Herman JM, Ray ME, Baker LH, Adsay NV, Rabah R, Lucas DR. Primary versus radiation-associated craniofacial osteosarcoma: biologic and clinicopathologic comparisons. Cancer. 2006;107:554–62.
Cheng YS, Wright JM, Walstad WR, Finn MD. Osteosarcoma arising in Paget’s disease of the mandible. Oral Oncol. 2002;38:785–92.
Lee YY, Van Tassel P, Nauert C, Raymond AK, Edeiken J. Craniofacial osteosarcomas: plain film, CT, and MR findings in 46 cases. AJR Am J Roentgenol. 1988;150:1397–402.
Demicco EG, Deshpande V, Nielsen GP, Kattapuram SV, Rosenberg AE. Well-differentiated osteosarcoma of the jaw bones: a clinicopathologic study of 15 cases. Am J Surg Pathol. 2010;34:1647–55.
Baumhoer D. Bone-related lesions of the jaws. Surg Pathol Clin. 2017;10:693–704.
Guerin M, Thariat J, Ouali M, et al. A new subtype of high-grade mandibular osteosarcoma with RASAL1/MDM2 amplification. Hum Pathol. 2016;50:70–8.
Ferrari D, Codeca C, Battisti N, et al. Multimodality treatment of osteosarcoma of the jaw: a single institution experience. Med Oncol. 2014;31:171.
Baumhoer D, Brunner P, Eppenberger-Castori S, Smida J, Nathrath M, Jundt G. Osteosarcomas of the jaws differ from their peripheral counterparts and require a distinct treatment approach. Experiences from the DOESAK Registry. Oral Oncol. 2014;50:147–53.
Liang L, Zhang T, You Y, He Q, Fan Y, Liao G. An individual patient data meta-analysis on the effect of chemotherapy on survival in patients with craniofacial osteosarcoma. Head Neck. 2019;41:2016–23.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hameed, M., Horvai, A.E. & Jordan, R.C.K. Soft Tissue Special Issue: Gnathic Fibro-Osseous Lesions and Osteosarcoma. Head and Neck Pathol 14, 70–82 (2020). https://doi.org/10.1007/s12105-019-01094-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-019-01094-2