Abstract
Background
Salivary gland intraductal papillary mucinous neoplasm (SG IPMN) is a recently proposed entity characterized by a papillary-cystic proliferation of mucin-producing cells. Because of overlapping histologic features and a clonal AKT1 p.E17K variant, SG IPMN has been presumed to be a precursor or a low-grade subtype of mucinous adenocarcinoma. NKX3.1 is a tumor suppressor gene located on chromosome 8p and is a known immunohistochemical marker of prostate epithelium and mucinous acinar cells of the intraoral salivary glands.
Methods
We retrieved 12 SG IPMN cases, and performed histologic and genetic analysis. Given the association of SG IPMN with mucinous acinar cells, we also investigated the performance of NKX3.1 as a marker of this tumor entity.
Results
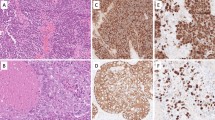
Diffuse and strong NKX3.1 expression was observed in all SG IPMN cases (12/12, 100%) as well as in normal mucinous acinar cells. In contrast, mucoepidermoid carcinoma and pancreatic IPMN cases as well as normal serous acinar cells were negative for NKX3.1. Genetically, 11 of 12 cases (92%) harbored an AKT1 p.E17K variant. A novel PTEN frameshift deletion (p.G36Dfs*18) was detected in the other single case. At least one of the histologic features implying malignant tumors, such as severe cellular atypia, brisk mitotic activity, high Ki-67 proliferating index, lymphovascular invasion, and lymph node metastasis, was detected in 6 SG IPMN cases (50%).
Conclusion
The findings suggest that SG IPMN is a low-grade subtype of mucinous adenocarcinoma which may be derived from mucinous acinar cells of the minor salivary gland.




Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, MN, upon reasonable request.
Code Availability
Not applicable.
References
Rooper LM, Agaimy A, Bishop JA, Gnepp D, Leivo I. Mucinous adenocarcinoma. In: WHO Classification of Tumours Online, Head and Neck Tumours (WHO classification of tumours series, 5th ed.; vol. 9). https://tumourclassification.iarc.who.int/chapters/52. Accessed 28 Apr 2022.
Rooper LM, Argyris PP, Thompson LDR, Gagan J, Westra WH, Jordan RC, et al. Salivary mucinous adenocarcinoma is a histologically diverse single entity with recurrent AKT1 E17K mutations: clinicopathologic and molecular characterization with proposal for a unified classification. Am J Surg Pathol. 2021;45(10):1337–47.
Agaimy A, Mueller SK, Bumm K, Iro H, Moskalev EA, Hartmann A, et al. Intraductal papillary mucinous neoplasms of minor salivary glands with AKT1 p.Glu17Lys mutation. Am J Surg Pathol. 2018;42(8):1076–82.
Nakaguro M, Urano M, Ogawa I, Hirai H, Yamamoto Y, Yamaguchi H, et al. Histopathological evaluation of minor salivary gland papillary-cystic tumours: focus on genetic alterations in sialadenoma papilliferum and intraductal papillary mucinous neoplasm. Histopathology. 2020;76(3):411–22.
Skálová A, Hyrcza MD, Leivo I. Update from the 5th edition of the World Health Organization classification of head and neck tumors: salivary glands. Head Neck Pathol. 2022;16(1):40–53.
Bishop JA. Proceedings of the North American Society of Head and Neck Pathology, Los Angeles, CA, March 20, 2022: emerging entities in salivary gland tumor pathology. Head Neck Pathol. 2022;16(1):179–89.
Griffin J, Chen Y, Catto JWF, El-Khamisy S. Gene of the month: NKX3.1. J Clin Pathol. 2022;75(6):361–4.
Schneider A, Brand T, Zweigerdt R, Arnold H. Targeted disruption of the Nkx3.1 gene in mice results in morphogenetic defects of minor salivary glands: parallels to glandular duct morphogenesis in prostate. Mech Dev. 2000;95(1–2):163–74.
Gurel B, Ali TZ, Montgomery EA, Begum S, Hicks J, Goggins M, et al. NKX3.1 as a marker of prostatic origin in metastatic tumors. Am J Surg Pathol. 2010;34(8):1097–105.
Tanaka M, Komuro I, Inagaki H, Jenkins NA, Copeland NG, Izumo S. Nkx3.1, a murine homolog of Ddrosophila bagpipe, regulates epithelial ductal branching and proliferation of the prostate and palatine glands. Dev Dyn. 2000;219(2):248–60.
Yoshida KI, Machado I, Motoi T, Parafioriti A, Lacambra M, Ichikawa H, et al. NKX3-1 is a useful immunohistochemical marker of EWSR1-NFATC2 sarcoma and mesenchymal chondrosarcoma. Am J Surg Pathol. 2020;44(6):719–28.
Takada N, Nishida H, Oyama Y, Kusaba T, Kadowaki H, Arakane M, et al. Immunohistochemical reactivity of prostate-specific markers for salivary duct carcinoma. Pathobiology. 2020;87(1):30–6.
Yang RK, Zhao P, Lu C, Luo J, Hu R. Expression pattern of androgen receptor and AR-V7 in androgen-deprivation therapy-naïve salivary duct carcinomas. Hum Pathol. 2019;84:173–82.
Agaimy A. Papillary neoplasms of the salivary duct system: a review. Surg Pathol Clin. 2021;14(1):53–65.
Zheng Z, Liebers M, Zhelyazkova B, Cao Y, Panditi D, Lynch KD, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20(12):1479–84.
Chu YH, Wirth LJ, Farahani AA, Nosé V, Faquin WC, Dias-Santagata D, et al. Clinicopathologic features of kinase fusion-related thyroid carcinomas: an integrative analysis with molecular characterization. Mod Pathol. 2020;33(12):2458–72.
Urano M, Nakaguro M, Yamamoto Y, Hirai H, Tanigawa M, Saigusa N, et al. Diagnostic significance of HRAS mutations in epithelial-myoepithelial carcinomas exhibiting a broad histopathologic spectrum. Am J Surg Pathol. 2019;43(7):984–94.
Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011;1:161.
Nakaguro M, Tada Y, Faquin WC, Sadow PM, Wirth LJ, Nagao T. Salivary duct carcinoma: updates in histology, cytology, molecular biology, and treatment. Cancer Cytopathol. 2020;128(10):693–703.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501.
Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–62.
Yang S, Zeng M, Chen X. Intraductal papillary mucinous neoplasm of the minor salivary gland with associated invasive micropapillary carcinoma. Am J Surg Pathol. 2019;43(10):1439–42.
Miura A, Mori T, Yoshida A, Watanabe Y, Sunami K, Watanabe S, et al. Primary adenocarcinoma of the trachea revealing a mucinous bronchial gland cell origin. Pathol Res Pract. 2018;214(5):796–9.
Acknowledgements
None
Funding
This study was funded by NIH/NHS 1P01CA240239-01 (WCF, PMS).
Author information
Authors and Affiliations
Contributions
MN, PMS, and WCF contributed to the study conception and design. Material preparation, data collection and analysis were performed by MN, PMS, RH, KK, TT, MU, TN, and WCF. The first draft of the manuscript was written by MN, PMS, and WCF. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The other authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
The present study was approved by the Institutional Review Board (IRB) of each collaborating institution (Mass General Brigham IRB 2015P001749, Nagoya University IRB 2018–019014195).
Consent to Participate
The need to obtain informed consent was waived due to the retrospective nature of the analysis.
Consent for Publication
The need to obtain informed consent was waived due to the retrospective nature of the analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakaguro, M., Sadow, P.M., Hu, R. et al. NKX3.1 Expression in Salivary Gland “Intraductal” Papillary Mucinous Neoplasm: A Low-Grade Subtype of Salivary Gland Mucinous Adenocarcinoma. Head and Neck Pathol 16, 1114–1123 (2022). https://doi.org/10.1007/s12105-022-01471-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-022-01471-4