Abstract
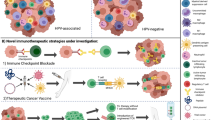
Cancer progression can be understood as the result of deregulation of tumors’ immune microenvironments. Recent studies of the alterations of microenvironments highlight their significant influence on the prognosis of patients with head and neck squamous cell carcinoma (HNSCC). It is necessary to better characterize tumor-infiltrating lymphocytes by focusing, in particular, on the tumor escape mechanisms from immune surveillance. One of the best described tumor immune system evasion mechanisms is the expression of co-stimulation molecules that constitute so-called “immune checkpoints”. These molecules regulate the immune response by either activating or inhibiting its effects. The programmed cell death 1 (PD-1) surface protein is an inhibitory co-stimulation molecule that induces exhaustion of activated T-lymphocytes (TLs, T cells) through binding with its ligands, PD-L1 and PD-L2. Half of HNSCCs exhibit PD-L1 expression with higher expression identified in human papillomavirus (HPV) positive tumors. Numerous studies have shown differences between the microenvironments of HPV+ and HPV− cancers. Notably, infiltrations of exhausted CD4+ PD1+ and CD8+ PD1+ T cells are far higher in the microenvironment of HPV+ tumors. The FDA has approved the use of molecules that target PD-1 for the treatment of HNSCC. The first results of clinical trials with anti-PD-1 blockers in HNSCC show improved patient survival, particularly long-term survival without recurrence. However, discordant results were sometimes observed, and improvements in defining cellular predictive markers are necessary. With the development of immunotherapies, pathologists play a role in the selection of patients who are eligible for specific treatments and assessment of their prognosis in greater detail. An automated, quantitative in situ imaging system that integrates both multispectral imaging and automated slide scanning could be developed in pathology laboratories. The evaluation of PD-L1 expression has only been used to stratify the administration of first-line immunotherapy. The validation of these tests and their routine interpretation is essential. No specific recommendation is adopted for HPV+ HNSCC.




Similar content being viewed by others
References
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Zhang Y, Huang S, Gong D, Qin Y, Shen Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol. 2010;7:389–95.
Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–38.
Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol (Baltimore, Md 1950). 2008;181:6738–46.
Sznol M, Chen L. Antagonist antibodies to PD-1 and B7–H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–34.
Outh-Gauer S, Alt M, Le Tourneau C, Augustin J, Broudin C, Gasne C, et al. Immunotherapy in head and neck cancers: a new challenge for immunologists, pathologists and clinicians. Cancer Treat Rev. 2018;65:54–64.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–18.
Badoual C. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–72.
Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6:1670–90.
She L, Qin Y, Wang J, Liu C, Zhu G, Li G, et al. Tumor-associated macrophages derived CCL18 promotes metastasis in squamous cell carcinoma of the head and neck. Cancer Cell Int. 2018;18:120.
Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18:269–82.
Zandberg DP, Strome SE. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50:627–32.
Yearley J, Gibson C, Yu N. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res An Off J Am Assoc Cancer Res. 2017;23:3158–67.
Ahmadi N, Gao K, Chia N, Kwon MS, Palme CE, Gupta R, et al. Association of PD-L1 expression in oral squamous cell carcinoma with smoking, sex, and p53 expression. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:631–8.
Ukpo OC, Thorstad WL, Lewis JS. B7–H1 expression model for immune evasion in human papillomavirus-related oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2013;7:113–21.
Moratin J, Metzger K, Safaltin A, Herpel E, Hoffmann J, Freier K, et al. Upregulation of PD-L1 and PD-L2 in neck node metastases of head and neck squamous cell carcinoma. Head Neck. 2019;41:2484–91.
Jie H-B, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629–35.
El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. In: World Health Organization classification of tumours. 4th ed. Lyon: International Agency for Research on Cancer; 2017.
Gillison ML, Broutian T, Pickard RKL, Tong Z, Xiao W, Kahle L, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307:693–703.
Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8.
Hayes DN, Grandis JR, El-Naggar AK. The Cancer Genome Atlas: integrated analysis of genome alterations in squamous cell carcinoma of the head and neck. J Clin Oncol. 2013;31:6009.
Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21:632–41.
Feldman R, Gatalica Z, Knezetic J, Reddy S, Nathan C-A, Javadi N, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E1625–1638.
Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22.
Jung AC, Briolat J, Millon R, de Reyniès A, Rickman D, Thomas E, et al. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int J Cancer. 2010;126:1882–944.
Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–41.
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37.
Partlová S, Bouček J, Kloudová K, Lukešová E, Zábrodský M, Grega M, et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology. 2015;4:e965570.
Cavalieri S, Rivoltini L, Bergamini C, Locati LD, Licitra L, Bossi P. Immuno-oncology in head and neck squamous cell cancers: news from clinical trials, emerging predictive factors and unmet needs. Cancer Treat Rev. 2018;65:78–86.
Mirghani H, Casiraghi O, Amen F, He M, Ma X-J, Saulnier P, et al. Diagnosis of HPV-driven head and neck cancer with a single test in routine clinical practice. Mod Pathol Springer Nature. 2015;28:1518–27.
Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393:51–60.
Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
Ruzevick J, Olivi A, Westra WH. Metastatic squamous cell carcinoma to the brain: an unrecognized pattern of distant spread in patients with HPV-related head and neck cancer. J Neurooncol. 2013;112:449–54.
Saloura V, Izumchenko E, Zuo Z, Bao R, Korzinkin M, Ozerov I, et al. Immune profiles in primary squamous cell carcinoma of the head and neck. Oral Oncol. 2019;96:77–88.
Tsao MS, Kerr KM, Kockx M, Beasley M-B, Borczuk AC, Botling J, et al. PD-L1 Immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018;13:1302–11.
Ratcliffe MJ, Sharpe A, Midha A, Barker C, Scott M, Scorer P, et al. Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non–small cell lung cancer. Clin Cancer Res. 2017;23:3585–91.
De Meulenaere A, Vermassen T, Creytens D, Aspeslagh S, Deron P, Duprez F, et al. Importance of choice of materials and methods in PD-L1 and TIL assessment in oropharyngeal squamous cell carcinoma. Histopathology. 2018;73:500–9.
Wang C, Hahn E, Slodkowska E, Eskander A, Enepekides D, Higgins K, et al. Reproducibility of PD-L1 immunohistochemistry interpretation across various types of genitourinary and head/neck carcinomas, antibody clones, and tissue types. Hum Pathol. 2018;82:131–9.
Hsu C, Lee S-H, Ejadi S, Even C, Cohen R, Le Tourneau C, et al. Antitumor activity and safety of pembrolizumab in patients with PD-L1-positive nasopharyngeal carcinoma: interim results from a phase 1b study. Ann Oncol. 2015;26:ix94.1–ix94.
FDA. FDA approves pembrolizumab for first-line treatment of head and neck squamous cell carcinoma [Internet]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-first-line-treatment-head-and-neck-squamous-cell-carcinoma. Accessed 20 Feb 2020.
Zandberg DP, Jarkowski A, Emeribe UA, Goswami T, Melillo G. A Phase 2, multicenter, single-arm, global study of MEDI4736 monotherapy in patients with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): HAWK (NCT02207530). J Clin Oncol. 2015;33:TPS6086.
Saâda-Bouzid E, Defaucheux C, Karabajakian A, Palomar Coloma V, Servois V, Paoletti X, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2017;28:1605–11.
Nizard M, Roussel H, Diniz MO, Karaki S, Tran T, Voron T, et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat Commun. 2017;8:15221.
Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16–related cancer. JAMA Oncol. 2019;5:67.
Dako. PD-L1 IHC 22C3 pharmDx interpretation manual—head and neck squamous cell carcinoma (HNSCC) [Internet]. https://www.agilent.com/cs/library/usermanuals/public/29314_22c3_pharmDx_hnscc_interpretation_manual_us.pdf. Accessed 20 Feb 2020.
Duncan DJ, Scott M, Scorer P, Barker C. Assessment of PD-L1 mRNA and protein expression in non-small cell lung cancer, head and neck squamous cell carcinoma and urothelial carcinoma tissue specimens using RNAScope and immunohistochemistry. Ahmad A, editor. PLoS ONE. 2019;14:e0215393.
Chen P-L, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 2016;6:827–37.
Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, et al. CD103+ tumor-resident CD8+ T cells are associated with improved survival in immunotherapy-naïve melanoma patients and expand significantly during anti-PD-1 treatment. Clin Cancer Res. 2018;24:3036–45.
Strome SE, Savva A, Brissett AE, Gostout BS, Lewis J, Clayton AC, et al. Squamous cell carcinoma of the tonsils: a molecular analysis of HPV associations. Clin Cancer Res An Off J Am Assoc Cancer Res. 2002;8:1093–100.
Kim HS, Lee JY, Lim SH, Park K, Sun J-M, Ko YH, et al. Association between PD-L1 and HPV status and the prognostic value of PD-L1 in oropharyngeal squamous cell carcinoma. Cancer Res Treat Off J Korean Cancer Assoc. 2016;48:527–36.
Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y, et al. Interferon-γ-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology. 2012;217:385–93.
Festino L, Botti G, Lorigan P, Masucci GV, Hipp JD, Horak CE, et al. Cancer treatment with anti-PD-1/PD-L1 agents: is PD-L1 expression a biomarker for patient selection? Drugs. 2016;76:925–45
Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103
Li X, Li M, Lian Z, Zhu H, Kong L, Wang P, et al. Prognostic role of programmed death ligand-1 expression in breast cancer: A systematic review and meta-analysis. Target Oncol. 2016;11:753–61.
Epacadostat shows value in two SCCHN trials. Cancer Discov. 2017;7:OF2. https://doi.org/10.1158/2159-8290.CD-NB2017-100.
Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–867.
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28.
Elbers JBW, Al-Mamgani A, Tesseslaar MET, van den Brekel MWM, Lange CAH, van der Wal JE, et al. Immuno-radiotherapy with cetuximab and avelumab for advanced stage head and neck squamous cell carcinoma: Results from a phase-I trial. Radiother Oncol. 2019;142:79–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Cécile Badoual has received research grants from Servier, has been member of advisory board BMS, Astrazeneca, MSD and has speaker honorarium from Merck, BMS, Roche, Astrazeneca. Eric Tartour has received research grants from GSK, Vaxeal, Servier, has been member of advisory board BMS, Astra-Zeneca and has speaker honorarium from Merck, BMS, Roche. Sophie Outh-Gauer, Aurélien Morini, Charles Lépine, Alain Jung declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Outh-Gauer, S., Morini, A., Tartour, E. et al. The Microenvironment of Head and Neck Cancers: Papillomavirus Involvement and Potential Impact of Immunomodulatory Treatments. Head and Neck Pathol 14, 330–340 (2020). https://doi.org/10.1007/s12105-020-01147-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-020-01147-x