Abstract
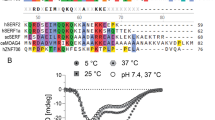
SASH1 is a scaffold protein with context-dependent biological functions in cell adhesion, tumor metastasis, lung development, and pigmentation. As a member of the SLy protein family, it contains the conserved SLY, SH3, and SAM domains. The 19 kDa SLY domain harbors over 70% of the SASH1 variants associated with pigmentation disorders. However, its solution structure or dynamics have not been investigated yet, and its exact position in the sequence is not clearly defined. Based on the bioinformatic and experimental evidence, we propose renaming this region to the SLy Proteins Associated Disordered Region (SPIDER) and defining the exact position to be amino acids 400–554 of SASH1. We have previously identified a variant in this region linked to a pigmentation disorder, S519N. Here, we used a novel deuteration technique, a suite of TROSY-based 3D NMR experiments, and a high-quality HNN to obtain near complete solution backbone assignment of SASH1’s SPIDER. A comparison with the chemical shifts of non-variant (S519) SPIDER shows that the S519N substitution does not alter the free form solution structural propensities of SPIDER. This assignment is the first step to characterize the role of SPIDER in SASH1-mediated cellular functions and provides a model for the future study of sister SPIDER domains in the SLy protein family.





Similar content being viewed by others
Data availability
The chemical shift assignment of the S519N variant of SPIDER (BMRB 51747) has been deposited in the Biological Magnetic Resonance Data Bank.
References
Araki A, Okamura K, Saito T (2021) Five novel mutations in SASH1 contribute to lentiginous phenotypes in Japanese families. Pigment Cell Melanoma Res 34:174–178
Beer S, Scheikl T, Reis B et al (2005) Impaired immune responses and prolonged allograft survival in sly1 mutant mice. Mol Cell Biol 25:9646–9660. https://doi.org/10.1128/mcb.25.21.9646-9660.2005
Cavanagh J (2007) Protein NMR spectroscopy: principles and practice. Academic Press, Cambridge
Chen S-Z, Zhang Y, Lei S-Y, Zhou F-Q (2020) SASH1 Suppresses the proliferation and invasion of human skin squamous cell carcinoma cells via inhibiting Akt cascade. OncoTarget Ther 30:4617–4625. https://doi.org/10.2147/OTT.S234667
Clements CM, Vögeli B, Shellman YG, Henen MA (2022) SAM1 domain of SASH1 harbors distinctive structural heterogeneity. J Struct Biol. https://doi.org/10.1016/j.jsb.2022.107914
Coulombe P, Paliouras GN, Clayton A et al (2019) Endothelial Sash1 is required for lung maturation through nitric oxide signaling. Cell Rep 27:1769–1780. https://doi.org/10.1016/j.celrep.2019.04.039
Courcet JB, Elalaoui SC, Duplomb L et al (2015) Autosomal-recessive SASH1 variants associated with a new genodermatosis with pigmentation defects, palmoplantar keratoderma and skin carcinoma. Eur J Hum Genet 23:957–962. https://doi.org/10.1038/ejhg.2014.213
Cui H, Guo S, He H et al (2020) SASH1 promotes melanin synthesis and migration via suppression of TGF-β1 secretion in melanocytes resulting in pathologic hyperpigmentation. Int J Biol Sci 16:1264–1273. https://doi.org/10.7150/ijbs.38415
Delaglio F, Grzesiek S, Vuister GW et al (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Franke FC, Müller J, Abal M et al (2019) The tumor suppressor SASH1 Interacts with the signal adaptor CRKL to inhibit epithelial–mesenchymal transition and metastasis in colorectal cancer. Cell Mol Gastroenterol Heptol 7:33–53. https://doi.org/10.1016/j.jcmgh.2018.08.007
Franke FC, Slusarenko BO, Engleitner T et al (2020) Novel role for CRK adaptor proteins as essential components of SRC/FAK signaling for epithelial-mesenchymal transition and colorectal cancer aggressiveness. https://doi.org/10.1002/ijc.32955
Hyberts SG, Milbradt AG, Wagner AB et al (2012) Application of iterative soft thresholding for fast reconstruction of NMR data non-uniformly sampled with multidimensional Poisson Gap scheduling. J Biomol NMR 52:315–327. https://doi.org/10.1007/s10858-012-9611-z
Ikura M, Marion D, Lewis, et al (1990) Heteronuclear 3D NMR and isotopic labeling of calmodulin: towards the complete assignment of the ’H NMR spectrum. Bio Chem Pharm 40(1):153–160
Jaufmann J, Franke FC, Sperlich A et al (2021) The emerging and diverse roles of the SLy/SASH1-protein family in health and disease—overview of three multifunctional proteins. FASEB J. https://doi.org/10.1096/fj.202002495r
Lee W, Tonelli M, Markley JL (2015) NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics 31:1325–1327. https://doi.org/10.1093/bioinformatics/btu830
Li J, Byrd RA (2022) A simple protocol for the production of highly deuterated proteins for biophysical studies. J Biol Chem. https://doi.org/10.1016/j.jbc.2022.102253
Lin S, Zhang J, Xu J et al (2012) Effects of SASH1 on melanoma cell proliferation and apoptosis in vitro. Mol Med Rep 6:1243–1248. https://doi.org/10.3892/mmr.2012.1099
Marsh JA, Singh VK, Jia Z, Forman-Kay JD (2006) Sensitivity of secondary structure propensities to sequence differences between α- and γ-synuclein: implications for fibrillation. Protein Sci 15:2795–2804. https://doi.org/10.1110/ps.062465306
Martini M, Gnann A, Scheikl D et al (2011) The candidate tumor suppressor SASH1 interacts with the actin cytoskeleton and stimulates cell-matrix adhesion. Int J Biochem Cell Biol 43:1630–1640. https://doi.org/10.1016/j.biocel.2011.07.012
Shellman YG, Lambert KA, Brauweiler A et al (2015) SASH1 is involved in an autosomal dominant lentiginous phenotype. J Invest Dermatol 135:3192–3194
Uchida T, Nakao A, Nakano N et al (2001) Identification of Nash1, a novel protein containing a nuclear localization signal, a sterile α motif, and an SH3 domain preferentially expressed in mast cells. Biochem Biophys Res Commun 288:137–141. https://doi.org/10.1006/bbrc.2001.5722
Vranken WF, Boucher W, Stevens TJ et al (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins: Struct Function Genet 59:687–696. https://doi.org/10.1002/prot.20449
Wang J, Zhang J, Li X et al (2017) A novel de novo mutation of the SASH1 gene in a chinese family with multiple lentigines. Acta Derm Venereol 97:530–531
Weidmann H (2015) SASH1, a new potential linkbetween smoking andatherosclerosis. Doctor of physiology and physiopathology. University Pierre et Marie Curie, Paris
Weidmann H, Touat-Hamici Z, Durand H et al (2015) SASH1, a new potential link between smoking and atherosclerosis. Atherosclerosis 242:571–579. https://doi.org/10.1016/j.atherosclerosis.2015.08.013
Weisemann R, Ruterjans H, Bermel W (1993) 3D Triple-resonance NMR techniques for the sequential assignment of NH and 15N resonances in 15N- and 13C-labelled proteins. J Biomol NMR 3:113–120. https://doi.org/10.1007/BF00242479
Zeller C, Hinzmann B, Seitz S et al (2003) SASH1: a candidate tumor suppressor gene on chromosome 6q24.3 is downregulated in breast cancer. Oncogene 22:2972–2983. https://doi.org/10.1038/sj.onc.1206474
Zhong WL, Wang HJ, Lin ZM, Yang Y (2019) Novel mutations in SASH1 associated with dyschromatosis universalis hereditaria. Indian J Dermatol Venereol Leprol 85:440
Zhou D, Wei Z, Deng S et al (2013) SASH1 regulates melanocyte transepithelial migration through a novel Gαs-SASH1-IQGAP1-E-Cadherin dependent pathway. Cell Signal 25:1526–1538. https://doi.org/10.1016/j.cellsig.2012.12.025
Zhou D, Wei Z, Kuang Z et al (2017) A novel P53/POMC/Gαs/SASH1 autoregulatory feedback loop activates mutated SASH1 to cause pathologic hyperpigmentation. J Cell Mol Med 21:802–815. https://doi.org/10.1111/jcmm.13022
Acknowledgements
The authors thank David Jones (University of Colorado, Denver) for his help and support.
Funding
The project was supported by NIH grants R01 AR074420 to YGS, R01 GM130694 to BV, and 1R21 AI171827 to MAH, University of Colorado Cancer Center Support Grant P30 CA046934, and NIH Biomedical Research Support Shared Grant S10 OD025020-01.
Author information
Authors and Affiliations
Contributions
All authors conceptualized the project and wrote the manuscript. CMC and MAH carried out experiments. CMC, YGS and MAH analyzed data. BV, YGS and MAH supervised the project and acquired funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent of publication
All authors have agreed to the publication of the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Clements, C.M., Vögeli, B., Shellman, Y.G. et al. Solution NMR backbone assignment of the SASH1 SLy proteins associated disordered region (SPIDER). Biomol NMR Assign 17, 151–157 (2023). https://doi.org/10.1007/s12104-023-10134-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-023-10134-6