Abstract
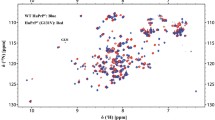
Human protein disulfide isomerase (PDI), a protein containing 4 domains a, b, b′, a′, disordered x linker and C-terminus, plays critical roles in disulfide bond reactions and proper protein folding in the endoplasmic reticulum. The bb′ domain contributes to client binding, the a, a′ domain catalyse the rearrangement of the disulfide bonds. The x linker and a′ domain were the main dynamics region for full-length PDI and the b′xa′ construct has the minimum functional domain within full-length PDI. Herein, we report a new preparation strategy with 1, 6-hexandiol and backbone NMR chemical shift assignments for the monomer b′xa′ domain.




Similar content being viewed by others
References
Aljanabi SM, Martinez I (1997) Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res 25:4692–4693. https://doi.org/10.1093/nar/25.22.4692
Ambadipudi S, Reddy JG, Biernat J, Mandelkow E, Zweckstetter M (2019) Residue-specific identification of phase separation hot spots of Alzheimer’s-related protein tau. Chem Sci 10:6503–6507. https://doi.org/10.1039/C9SC00531E
Byrne LJ, Sidhu A, Wallis AK, Ruddock LW, Freedman RB, Howard MJ, Williamson RA (2009) Mapping of the ligand-binding site on the b’ domain of human PDI: interaction with peptide ligands and the x-linker region. Biochem J 423:209–217. https://doi.org/10.1042/bj20090565
Darby NJ, Creighton TE (1995) Functional properties of the individual thioredoxin-like domains of protein disulfide isomerase. Biochemistry 34:11725–11735. https://doi.org/10.1021/bi00037a009
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293. https://doi.org/10.1007/BF00197809
Dijkstra K et al (1999) Assignment of 1H, 13C and 15N resonances of the a’ domain of protein disulfide isomerase. J Biomol NMR 14:195–196. https://doi.org/10.1023/a:1008331225208
Goddard T, Kneller D (2004) SPARKY 3. University of California, San Francisco
Klappa P, Ruddock LW, Darby NJ, Freedman RB (1998) The b′ domain provides the principal peptide-binding site of protein disulfide isomerase but all domains contribute to binding of misfolded proteins. EMBO J 17:927–935. https://doi.org/10.1093/emboj/17.4.927
Nguyen VD et al (2008) Alternative conformations of the x region of human protein disulphide-isomerase modulate exposure of the substrate binding b’ domain. J Mol Biol 383:1144–1155. https://doi.org/10.1016/j.jmb.2008.08.085
Okumura M et al (2019) Dynamic assembly of protein disulfide isomerase in catalysis of oxidative folding. Nat Chem Biol 15:499–509. https://doi.org/10.1038/s41589-019-0268-8
Patel SS, Belmont BJ, Sante JM, Rexach MF (2007) Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell 129:83–96. https://doi.org/10.1016/j.cell.2007.01.044
Ribbeck K, Görlich D (2002) The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J 21:2664–2671. https://doi.org/10.1093/emboj/21.11.2664
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44:213–223. https://doi.org/10.1007/s10858-009-9333-z
Tian G, Kober F-X, Lewandrowski U, Sickmann A, Lennarz WJ, Schindelin H (2008) The catalytic activity of protein-disulfide isomerase requires a conformationally flexible molecule. J Biol Chem 283:33630–33640. https://doi.org/10.1074/jbc.M806026200
Tian G, Xiang S, Noiva R, Lennarz WJ, Schindelin H (2006) The crystal structure of yeast protein disulfide isomerase suggests cooperativity between its active sites. Cell 124:61–73. https://doi.org/10.1016/j.cell.2005.10.044
Wallis AK, Sidhu A, Byrne LJ, Howard MJ, Ruddock LW, Williamson RA, Freedman RB (2009) The ligand-binding b’ domain of human protein disulphide-isomerase mediates homodimerization. Protein Sci 18:2569–2577. https://doi.org/10.1002/pro.270
Wang C, Chen S, Wang X, Wang L, Wallis AK, Freedman RB, Wang C-c (2010) Plasticity of human protein disulfide isomerase: evidence for mobility around the x-linker region and its functional significance. J Biol Chem 285:26788–26797. https://doi.org/10.1074/jbc.M110.107839
Wang C et al (2013a) Structural insights into the redox-regulated dynamic conformations of human protein disulfide isomerase. Antioxid Redox Signal 19:36–45. https://doi.org/10.1089/ars.2012.4630
Wang C, Yu J, Huo L, Wang L, Feng W, Wang CC (2012) Human protein-disulfide isomerase is a redox-regulated chaperone activated by oxidation of domain a’. J Biol Chem 287:1139–1149. https://doi.org/10.1074/jbc.M111.303149
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21773300, 21925406).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pei, Y., Liu, X., Cheng, K. et al. Backbone resonance assignment of PDI b'xa' domain construct. Biomol NMR Assign 15, 409–413 (2021). https://doi.org/10.1007/s12104-021-10038-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-021-10038-3