Abstract
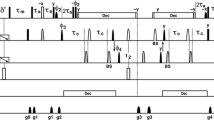
To determine the three-dimensional solution structure of the calcium bound S100P protein, the backbone and side chain resonance assignments of the S100P protein have been reported based on triple-resonance experiments using uniformly [13C, 15N]-labeled calcium bound protein.


Similar content being viewed by others
References
Arumugam T, Logsdon CD (2010) S100P: a novel therapeutic target for cancer. Amino Acids. doi:10.1007/s00726-010-0496-4
Arumugam T, Simeone DM, Schmidt AM, Logsdon CD (2004) S100P stimulates cell proliferation and survival via receptor for activated glycation end products (RAGE). J Biol Chem 279:5059–5065
Cornilescu G, Delagio F, Bax A (1999) Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J Biomol NMR 13:289–302
Fuentes MK, Nigavekar SS, Arumugam T, Logsdon CD, Schmidt AM, Park JC, Huang EH (2007) RAGE activation by S100P in colon cancer stimulates growth, migration, and cell signalingpathways. Dis Colon Rectum 50(8):1230–1240
Marenholz I, Heizmann CW, Fritz G (2004) S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun 322:1111–1122
Marenholz I, Lovering RC, Heizmann CW (2006) An update of the S100 nomenclature. Biochim Biophys Acta 1763:1282–1283
Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT (2009) RAGE (receptor for advanced glycation endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med 7:17 (Review)
Wishart DS, Sykes BD (1994) The 13C chemical shift index: as simple method for the identification of protein secondary structure using 13C chemical shift data. J Biomol NMR 4:171–180
Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, Oldfield E, Markley JL, Sykes BD (1995) 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J Biomol NMR 6:135–140
Acknowledgments
Work in our laboratory is supported by Grant from the National Science Council (NSC) Taiwan (NSC 100-2113-M-007-012-MY3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Penumutchu, S.R., Mohan, S.K. & Yu, C. 1H, 15N and 13C assignments of the calcium bound S100P. Biomol NMR Assign 7, 5–8 (2013). https://doi.org/10.1007/s12104-012-9365-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-012-9365-2