Abstract
Ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), also known as PGP9.5, is a protein of 223 amino acids. Although it was originally characterized as a deubiquitinating enzyme, recent studies indicate that it also functions as a ubiquitin (Ub) ligase and a mono-Ub stabilizer. It is highly abundant in brain, constituting up to 2% of total brain proteins. Down-regulation and extensive oxidative modification of UCH-L1 have been observed in the brains of Alzheimer’s disease (AD) and Parkinson’s disease (PD) patients. Mutations in the UCH-L1 gene have been reported to be linked to Parkinson’s disease, in particular, the I93 M variant is associated with a higher susceptibility of PD in contrast to a higher protection against PD for the S18Y variant. Hence, the structure of UCH-L1 and the underlying effects of disease associated mutations on the structure and function of UCH-L1 are of considerable interest. Here, we report the NMR spectral assignments of the S18Y human UCH-L1 mutant with the aim to obtain better understanding about the risk of Parkinson’s disease against structural and dynamical changes induced by this mutation on UCH-L1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Biological context
The mechanism of protein metabolism in living cells through the proteasome system, ubiquitination, and deubiquitination is an important biological process. In particular, deubiquitination is considered essential for negative regulation of proteolysis and for recycling of ubiquitin from polyubiquitin chains. Ubiquitin C-terminal hydrolase (UCH-L1), a multifunctional 24.8 kDa protein, is one of the most abundant proteins in the brain (1–2% of the total soluble protein) (Wilkinson et al. 1989). Mutations in the UCH-L1 gene have been reported to be linked to susceptibility to and protection from Parkinson’s disease (PD) (Leroy et al. 1998; Maraganore et al. 1999). Furthermore, abnormal overexpression of UCH-L1 has been shown to correlate with several forms of cancer (Hibi et al. 1998). The I93 M UCH-L1 mutant linked to higher susceptibility to PD was found to cause the accumulation of α-synuclein in cultured cells, which is an effect that cannot be explained by its originally recognized deubiquitinating hydrolase activity. Subsequently, UCH-L1 was shown to exhibit an unexpected concentration dependent ubiquitin ligase activity (Liu et al. 2002) and a mono-ubiquitin stabilizer activity (Osaka et al. 2003). On the contrary, a polymorphic variant of UCH-L1, S18Y, that is associated with decreased PD risk has been shown to have a reduced ligase activity but comparable hydrolase activity as the wild-type enzyme. Thus, the ligase activity as well as the hydrolase activity of UCH-L1 may play a role in proteasomal protein degradation in the brain, which is a critical process for neuronal health.
The amino acid sequence of UCH-L1 is similar to that of other ubiquitin C-terminal hydrolases, including the ubiquitously expressed UCH-L3, which shares the conserved catalytic residues and 51% sequence identity with UCH-L1. But UCH-L3 appears to be unconnected to neurodegenerative disease. Therefore, the structure of UCH-L1 and the effects of disease associated mutations on the structure and function of UCH-L1 are of considerable interest and importance. Indeed, a recent small-angle neutron scattering study indicated that the I93 M mutation promotes ellipsoidal deformation by a decrease of the β-turn contents whereas the S18Y mutation recovers the globularity of the protein by an increase of β-turns (Naito et al. 2006). As a first step to provide detailed structural information on molecular level and enable greater understanding of how the structure of these UCH-L1 mutants relates to the etiology of PD, we present here the backbone and side chain assignments of the S18Y human UCH-L1 mutant.
Methods and experiments
Cloning, expression, and purification
Full-length UCH-L1 was cloned into a pET-22b vector by using standard cloning protocols. The resulting C-terminal (His)6-tagged protein was expressed in Escherichia coli strain BL21(DE3) (Novagen) using M9 minimal media supplemented with 2.0 g/l [U-13C] glucose and 1.0 g/l 15NH4Cl (Cambridge Isotopes Laboratory) as the sole nitrogen and carbon sources. Bacterial cultures were grown at 37°C to an optical density of 0.6–0.7. After 0.2 mM IPTG induction at 22°C for 16 h, the protein was then purified with a nickel-nitrilotriacetic acid column (Qiagen) following the manufacturer’s recommendations. The protein was further purified by size exclusion chromatography on a HiLoad 16/60 Superdex 75 prep grade column (Amersham Pharmacia). The purity of the (His)6-tagged protein was estimated to be over 95% by SDS–PAGE.
NMR experiments
The samples were prepared in a buffer containing 20 mM sodium phosphate, pH 6.5; 100 mM NaCl; 2 mM DTT and 1 mM PMSF in 90% H2O/10% D2O. All NMR experiments were conducted at 298 K on a Bruker Advance 600 MHz NMR spectrometer equipped with a TCI cryoprobe. A 2D {15N,1H}-HSQC and a series of triple resonance experiments including 3D HNCACB, 3D CBCA(CO)NH, 3D HNCO, 3D HNCACO, 3D HNCA, 3D HNCOCA, 3D HBHA(CO)NH as well as 3D HCCH-TOCSY and 3D HCCH-COSY experiments have been carried out to allow sequence specific backbone and side-chains resonance assignments of the protein. Trimethylsilyl propanoate (TSP) was used as a chemical shift reference for 1H while 13C and 15N chemical shifts were referenced indirectly, using the gyromagnetic ratios of 15N, 13C and 1H (15N/1H = 0.101329118, 13C/1H = 0.251449530). All NMR spectra were processed using XWINNMR software (Bruker GmbH). The chemical shifts of backbone and side-chain resonances were assigned manually using SPARKY program (Goddard and Kneller 1993).
Assignments and data deposition
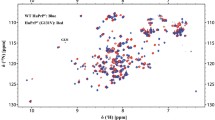
A 2D 1H-15N HSQC spectrum of UCH-L1-S18Y, with assignment indicated, is shown in Fig. 1. The peaks observed in this spectrum is well-dispersed, indicating that the protein is well structured. All expected backbone 1H-15N correlations have been assigned, with the exception of Q84 and F162. The hexa-His tag at the C-terminus is also unassigned due to considerable signal overlap. In total, 90.5% of all protons and 84.5% of all aliphatic carbons have been assigned. All 1H, 15N and 13C backbone and side-chain chemical shift assignments have been deposited at the BMRB (http://www.bmrb.wisc.edu) and can be accessed under the accession number 17260. Analysis of the 1Hα, 13Cα, 13Cβ and 13C’ chemical shift using Chemical Shift Index (Wishart and Sykes 1994) is summarized in Fig. 2. The predicted secondary structure of UCH-L1-S18Y is largely consistent with that found in the crystal structure of wild type UCH-L1 (Das et al. 2006).
1H–15N HSQC spectrum acquired at 298 K on a Bruker Avance 600 MHz spectrometer showing the assignments for UCH-L1-S18Y. The backbone 1H–15N correlations are sequence-specifically labeled and the Asn and Gln side-chain NH2 peaks are linked by horizontal solid lines. The central region of the spectrum a is expanded and shown in b for clarity
Secondary structure prediction of UCH-L1-S18Y. Indicated underneath the amino acid sequence of UCH-L1-S18Y are the carton representation of the positions of the sheets and helical regions observed in the crystal structure of wild type UCH-L1 (blue) and the secondary structure prediction of UCH-L1-S18Y by CSI (red). Arrows represent the helices and waves the sheets
References
Das C, Hoang QQ, Kreinbring CA, Luchansky SJ, Meray RK, Ray SS, Lansbury PT, Ringe D, Petsko GA (2006) Structural basis for conformational plasticity of the Parkinson’s disease-associated ubiquitin hydrolase UCH-L1. Proc Natl Acad Sci USA 103:4675–4680
Goddard TD, Kneller DG (1993) SPARKY 3. University of California, San Francisco
Hibi K, Liu Q, Beaudry GA et al (1998) Serial analysis of gene expression in non-small cell lung cancer. Cancer Res 58:5690–5694
Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E et al (1998) The ubiquitin pathway in Parkinson’s disease. Nature 395:451–452
Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT Jr (2002) The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell 111:209–18
Maraganore DM, Farrer MJ, Hardy JA, McDonnell SK, Lincoln SJ, Rocca WA (1999) Case-control study of the ubiquitin carboxy-terminal hydrolase L1 gene in Parkinson’s disease. Neurology 53:1858–1860
Naito S, Mochizuki H, Yasuda T et al (2006) Characterization of multimetric variants of ubiquitin carboxyl-terminal hydrolase L1 in water by small-angle neutron scattering. Biochem Biophys Res Commun 339:717–725
Osaka H, Wang YL, Takada K et al (2003) Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Hum Mol Genet 12:1945–1958
Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J (1989) The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science 246:670–673
Wishart DS, Sykes BD (1994) The13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR 4:171–180
Acknowledgments
This work was supported by the Research Grants Council of Hong Kong (GRF 7755/08 M, 7765/09 M)
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Tse, HS., Hu, HY. & Sze, KH. Backbone and side-chain 1H, 15N and 13C resonance assignments of S18Y mutant of ubiquitin carboxy-terminal hydrolase L1. Biomol NMR Assign 5, 165–168 (2011). https://doi.org/10.1007/s12104-011-9292-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-011-9292-7