Abstract
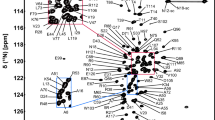
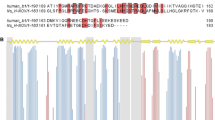
Rhodanese domain is a ubiquitous structural module commonly found in bacterial, archaeal and eukaryotic cells. Growing evidence indicates that rhodanese domains act as the carrier of reactive sulfur atoms by forming persulfide intermediates in distinct metabolic pathways. YgaP, a membrane protein consisting of a rhodanese domain and a C-terminal transmembrane segment, is the only membrane-associated rhodanese in Escherichia coli. Herein, we report the resonance assignments of 1H, 13C and 15N atoms of rhodanese domain of YgaP. Totally, chemical shifts of more than 95% of the atoms were assigned.


Similar content being viewed by others
References
Bordo D, Bork P (2002) The rhodanese/Cdc25 phosphatase superfamily Sequence-structure-function relations. EMBO Rep 3:741–746. doi:10.1093/embo-reports/kvf150
Cipollone R, Ascenzi P, Visca P (2007) Common themes and variations in the rhodanese superfamily. IUBMB Life 59:51–59. doi:10.1080/15216540701206859
Delaglio F, Grzesiek S, Vuister GW et al (1995) NMRPipe: a multidimensional spectral processing system based on Unix pipes. J Biomol NMR 6:277–293. doi:10.1007/BF00197809
Johnson BA, Blevins RA (1994) NMRView: a computer program for the visualization and analysis of NMR data. J Biomol NMR 4:603–614. doi:10.1007/BF00404272
Marley J, Lu M, Bracken C (2001) A method for efficient isotopic labeling of recombinant proteins. J Biomol NMR 20:71–75. doi:10.1023/A:1011254402785
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Magn Reson Spectrosc 34:93–158. doi:10.1016/S0079-6565(98)00025-9
Spallarossa A, Donahue JL, Larson TJ, Bolognesi M, Bordo D (2001) Escherichia coli GlpE is a prototype sulfurtransferase for the single-domain rhodanese homology superfamily. Structure 9:1117–1125. doi:10.1016/S0969-2126(01)00666-9
Wishart DS, Sykes BD (1994) The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR 4:171–180. doi:10.1007/BF00175245
Acknowledgments
All NMR experiments were carried out at the Beijing NMR Center. This work was supported by Grant 2006CB910203 from the National Basic Research Program and Grant 2006AA02A323 from the National High Technology Research and Development Program of China (to C.J.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Bi, Y., Xia, B. et al. 1H, 13C and 15N resonance assignments of the rhodanese domain of YgaP from Escherichia coli . Biomol NMR Assign 5, 101–103 (2011). https://doi.org/10.1007/s12104-010-9277-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-010-9277-y