Abstract
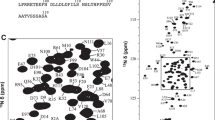
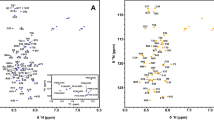
The human AKAP13 protein contains DH and PH domains, which are responsible for its cell transforming activity. Despite its biomedical importance, the contribution of the PH domain to AKAP13 activity remains unclear and no three dimensional structure is available to date. Here we report the backbone and side chain 1H, 13C and 15N resonance assignments of a 20 kDa construct comprising the uniformly 13C and 15N labeled AKAP13-PH domain and an associated helix from the DH domain which is required for its stable expression. Resonance assignment has been achieved using conventional triple resonance experiments; 95% of all back bone resonances and more than 90% of side chain resonances have been successfully assigned. The 1H, 13C and 15N chemical shifts have been deposited in BMRB with accession number of 16195.


Similar content being viewed by others
Abbreviations
- AKAP13:
-
A kinase anchor protein 13
- Brx:
-
Breast cancer nuclear receptor-binding auxiliary protein
- DH:
-
Dbl homology
- EDTA:
-
Ethylenediaminetetraacetic acid
- GEF:
-
Guanine nucleotide exchange factor
- Lbc:
-
Lymphoid blast crisis
- PH domain:
-
Pleckstrin homology
- PKA:
-
Protein kinase A
References
Auguin D, Barthe P, Augé-Sénégas MT, Stern MH, Noguchi M, Roumestand C (2004) Solution structure and backbone dynamics of the pleckstrin homology domain of the human protein kinase B (PKB/Akt). Interaction with inositol phosphates. J Biomol NMR 28:137–155
Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, Diviani D, Bristow MR, Kunkel MT, Newton AC, Langeberg LK, Scott JD (2008) AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell 32:169–179
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Diviani D, Baisamy L, Appert-Collin A (2006) AKAP-Lbc: a molecular scaffold for the integration of cyclic AMP and Rho transduction pathways. Eur J Cell Biol 85:603–610
Maffucci T, Falasca M (2001) Specificity in pleckstrin homology (PH) domain membrane targeting: a role for a phosphoinositide-protein co-operative mechanism. FEBS Lett 506:173–179
Shibolet O, Giallourakis C, Rosenberg I, Mueller T, Xavier RJ, Podolsky DK (2007) AKAP13, a RhoA GTPase-specific guanine exchange factor, is a novel regulator of TLR2 signaling. J Biol Chem 282:35308–35317
Sondermann H, Soisson SM, Boykevisch S, Yang SS, Bar-Sagi D, Kuriyan J (2004) Structural analysis of autoinhibition in the Ras activator Son of sevenless. Cell 119:393–405
Sterpetti P, Hack AA, Bashar MP, Park B, Cheng SD, Knoll JH, Urano T, Feig LA, Toksoz D (1999) Activation of the Lbc Rho exchange factor proto-oncogene by truncation of an extended C terminus that regulates transformation and targeting. Mol Cell Biol 19:1334–1345
Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59:687–696
Worthylake DK, Rossman KL, Sondek J (2004) Crystal structure of the DH/PH fragment of Dbs without bound GTPase. Structure 12:1078–1086
Acknowledgments
We thank all the staff of The Henry Wellcome Building for Biological NMR Spectroscopy, which is funded by the Wellcome Trust. This work was funded by Cancer Research UK and EU PRISM [M.O.] and carried out in collaboration with the Structural Genomics Consortium, a registered charity (number 1097737) funded by the Wellcome Trust, GlaxoSmithKline, Genome Canada, the Canadian Institutes of Health Research, the Ontario Innovation Trust, the Ontario Research and Development Challenge Fund and the Canadian Foundation for Innovation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugawara, M., Whittaker, S.BM., Bishop, S. et al. Resonance assignments of the human AKAP13-PH domain and stabilizing DH helix. Biomol NMR Assign 3, 215–218 (2009). https://doi.org/10.1007/s12104-009-9178-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-009-9178-0