Abstract
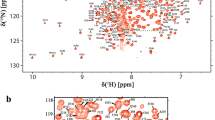
SUMO, an important post-translational modifier of variety of substrate proteins, regulates different cellular functions. Here, we report the NMR resonance assignment of the folded and 8 M urea-denatured state of SUMO from Drosophila melanogaster (dsmt3).



Similar content being viewed by others
References
Alarcon-Vargas D, Ronai Z (2002) SUMO in cancer-wrestlers wanted. Cancer Biol Ther 1:237–242
Baldi P, Brunak S, Frasconi P, Soda G, Pollastri G (1999) Exploiting the past and the future in protein secondary structure prediction. Bioinformatics 15:937–946
Bhaskar V, Valentine SA, Courey AJ (2000) A functional interaction between dorsal and components of the Smt3 conjugation machinery. J Biol Chem 275:4033–4040
Chatterjee A, Bhavesh NS, Panchal SC, Hosur RV (2002) A novel protocol based on HN(C)N for rapid resonance assignment in ((15)N, (13)C) labeled proteins: implications to structural genomics. Biochem Biophy Res Commun 293:427–432
Keller R (2004), The computer aided resonance assignment tutorial. CANTINA Verlag Goldau
Lehembre F, Muller S, Pandolfi PP, Dejean A (2001) Regulation of Pax3 transcriptional activity by SUMO-1-modified PML. Oncogene 20(1):1–9
Melchior F (2000) SUMO—nonclassical ubiquitin. Annu Rev Cell Dev Biol 16:591–626
Panchal SC, Bhavesh NS, Hosur RV (2001) Improved 3D triple resonance experiments, HNN and HN(C)N, for HN and 15N sequential correlations in (13C, 15N) labeled proteins: application to unfolded proteins. J Biomol NMR 20:135–147
Seeler JS, Dejean A (2001) SUMO: of branched proteins and nuclear bodies. Oncogene 20:7243–7249
Verger A, Perdomo J, Crossley M (2003) Modification with SUMO. A role in transcriptional regulation. EMBO Rep 4:137–142
Wishart DS, Bigam CG, Holm A, Hodges RS, Sykes BD (1995) 1H, 13C and 15N random coil NMR chemical shifts of the common amino acids. I. Investigations of nearest-neighbor effects. J Biomol NMR 5:67–81
Acknowledgement
We thank the Government of India for providing financial support to the National Facility for High Field NMR at the Tata Institute of Fundamental Research, INDIA. DK acknowledges Fred Damberger for all the help provided for learning the software CARA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, D., Kumar, A., Misra, J.R. et al. 1H, 15N, 13C resonance assignment of folded and 8 M urea-denatured state of SUMO from Drosophila melanogaster . Biomol NMR Assign 2, 13–15 (2008). https://doi.org/10.1007/s12104-007-9072-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-007-9072-6