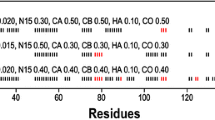
Abstract
Ubiquitin signaling in eukaryotes is responsible for a variety of cellular outcomes, most notably proteasomal degradation. A recent bioinformatic study has revealed the existence of a new proteasomal operon in certain gram-negative bacteria phyla. This operon contains genes similar to those included in the prokaryotic ubiquitin-like protein (Pup) proteasomal operon, but do not themselves contain Pup. Instead, they encode for a protein termed UBact with 30% sequence similarity to Pup. Here, we report the near-complete NMR assignment of the backbone and partial assignment of the side chain chemical shifts of the UBact protein from Nitrospira nitrosa. The 1H–15N HSQC spectrum shows a narrow spread of proton NMR signals, characteristic of an intrinsically disordered protein. This chemical shift assignment will facilitate further NMR studies to explore the role of UBact in this new putative proteasomal operon.




Similar content being viewed by others
Data availability
The assignment data were deposited in the BioMagResBank, entry number 51116.
Code availability
Not applicable.
Consent for publication
Not applicable.
References
Barandun J, Damberger FF, Delley CL, Laederach J, Allain FH, Weber-Ban E (2017) Prokaryotic ubiquitin-like protein remains intrinsically disordered when covalently attached to proteasomal target proteins. BMC Struct Biol 17(1):1. https://doi.org/10.1186/s12900-017-0072-1
Benoist P, Müller A, Diem HG, Schwencke J (1992) High-molecular-mass multicatalytic proteinase complexes produced by the nitrogen-fixing actinomycete Frankia strain BR. J Bacteriol 174(5):1495–1504. https://doi.org/10.1128/jb.174.5.1495-1504.1992
Bode NJ, Darwin KH (2014) The Pup-proteasome system of Mycobacteria. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.MGM2-0008-2013
Dahlmann B, Kopp F, Kuehn L, Niedel B, Pfeifer G, Hegerl R, Baumeister W (1989) The multicatalytic proteinase (prosome) is ubiquitous from eukaryotes to archaebacteria. FEBS Lett 251(1–2):125–131
Grzesiek S, Bax A (1993) The importance of not saturating water in protein NMR. Application to sensitivity enhancement and NOE measurements. J Am Chem Soc 115(26):12593–12594
Hall JB, Fushman D (2003) Characterization of the overall and local dynamics of a protein with intermediate rotational anisotropy: differentiating between conformational exchange and anisotropic diffusion in the B3 domain of protein G. J Biomol NMR 27(3):261–275. https://doi.org/10.1023/a:1025467918856
Hough R, Pratt G, Rechsteiner M (1987) Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J Biol Chem 262(17):8303–8313
Kjaergaard M, Poulsen FM (2011) Sequence correction of random coil chemical shifts: correlation between neighbor correction factors and changes in the Ramachandran distribution. J Biomol NMR 50(2):157–165. https://doi.org/10.1007/s10858-011-9508-2
Kjaergaard M, Brander S, Poulsen FM (2011) Random coil chemical shift for intrinsically disordered proteins: effects of temperature and pH. J Biomol NMR 49(2):139–149. https://doi.org/10.1007/s10858-011-9472-x
Lee W, Tonelli M, Markley JL (2015) NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics 31(8):1325–1327. https://doi.org/10.1093/bioinformatics/btu830
Lehmann G, Udasin RG, Livneh I, Ciechanover A (2017) Identification of UBact, a ubiquitin-like protein, along with other homologous components of a conjugation system and the proteasome in different gram-negative bacteria. Biochem Biophys Res Commun 483(3):946–950. https://doi.org/10.1016/j.bbrc.2017.01.037
Li Y, Maciejewski MW, Martin J, Jin K, Zhang Y, Maupin-Furlow JA, Hao B (2013) Crystal structure of the ubiquitin-like small archaeal modifier protein 2 from Haloferax volcanii. Protein Sci 22(9):1206–1217. https://doi.org/10.1002/pro.2305
Maupin-Furlow JA (2013) Archaeal proteasomes and SAMPylation. Subcell Biochem 66:297–327. https://doi.org/10.1007/978-94-007-5940-4_11
Miranda HV, Nembhard N, Su D, Hepowit N, Krause DJ, Pritz JR, Phillips C, Soll D, Maupin-Furlow JA (2011) E1- and ubiquitin-like proteins provide a direct link between protein conjugation and sulfur transfer in archaea. Proc Natl Acad Sci USA 108(11):4417–4422. https://doi.org/10.1073/pnas.1018151108
Pickart CM, Fushman D (2004) Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol 8(6):610–616. https://doi.org/10.1016/j.cbpa.2004.09.009
Ranjan N, Damberger FF, Sutter M, Allain FH, Weber-Ban E (2011) Solution structure and activation mechanism of ubiquitin-like small archaeal modifier proteins. J Mol Biol 405(4):1040–1055. https://doi.org/10.1016/j.jmb.2010.11.040
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44(4):213–223. https://doi.org/10.1007/s10858-009-9333-z
Sutter M, Damberger FF, Imkamp F, Allain FH, Weber-Ban E (2010) Prokaryotic ubiquitin-like protein (Pup) is coupled to substrates via the side chain of its C-terminal glutamate. J Am Chem Soc 132(16):5610–5612. https://doi.org/10.1021/ja910546x
Venyaminov SYu, Baikalov IA, Shen ZM, Wu CS, Yang JT (1993) Circular dichroic analysis of denatured proteins: inclusion of denatured proteins in the reference set. Anal Biochem 214(1):17–24. https://doi.org/10.1006/abio.1993.1450
Vijay-Kumar S, Bugg CE, Cook WJ (1987) Structure of ubiquitin refined at 1.8 A resolution. J Mol Biol 194(3):531–544. https://doi.org/10.1016/0022-2836(87)90679-6
Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Markley J, Ionides J, Laue ED (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59(4):687–696. https://doi.org/10.1002/prot.20449
Wiedemann C, Bellstedt P, Görlach M (2013) CAPITO—a web server-based analysis and plotting tool for circular dichroism data. Bioinformatics 29(14):1750–1757. https://doi.org/10.1093/bioinformatics/btt278
Acknowledgements
Supported by the National Institutes of Health Grant GM065334 to DF. NMR experiments were performed on instruments supported in part by the National Science Foundation Grant DBI1040158. SMB thanks Westley Pawloski for discussion and Ananya Majumdar for HN(CO)CA pulse sequence.
Funding
Supported by a Grant from the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
SMB performed experiments, analyzed data, and wrote the manuscript. DF oversaw the project and assisted in writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bonn, S.M., Fushman, D. Backbone NMR resonance assignment of the intrinsically disordered UBact protein from Nitrospira nitrosa. Biomol NMR Assign 16, 129–134 (2022). https://doi.org/10.1007/s12104-022-10070-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-022-10070-x