Abstract
Acute kidney injury (AKI) is common in critically ill patients, affecting almost one in four critically ill children and one in three neonates. Higher stages of AKI portend worse outcomes. Identifying AKI timely and instituting appropriate measures to prevent and manage severe AKI is important, since it is independently associated with mortality. Methods to predict severe AKI should be applied to all critically ill patients. Assessment of volume status to prevent the development of fluid overload is useful to prevent adverse outcomes. Patients with metabolic or clinical complications of AKI need prompt kidney replacement therapy (KRT). Various modes of KRT are available, and the choice of modality depends most on the technical competence of the center, patient size, and hemodynamic stability. Given the significant risk of chronic kidney disease, patients with AKI require long-term follow-up. It is important to focus on improving awareness about AKI, incorporate AKI prevention as a quality initiative, and improve detection, prevention, and management of AKI with the aim of reducing acute and long-term morbidity and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is a clinical syndrome characterized by an abrupt decrease in kidney function, resulting in the accumulation of waste products and critical imbalances in fluid and electrolyte homeostasis. The term ‘AKI’ replaced ‘acute renal failure’ to encompass kidney dysfunction ranging from mildly elevated serum creatinine to severe forms requiring kidney replacement therapy (KRT) [1], emphasizing that even small increments in creatinine are associated with adverse short- and long-term outcomes.
Definitions and Staging
AKI has conventionally been defined and staged for severity based on serum creatinine and urine output (Table 1). The KDIGO criteria, which harmonize the AKIN with the RIFLE criteria, are most commonly used in adults and children [2]. The pediatric reference change value optimized criterion for AKI (pROCK), which is based on paired creatinine values in Chinese children admitted with nonrenal morbidities [3], might be more specific than KDIGO criteria in detecting ‘true’ AKI, but requires validation.
Neonatal AKI is more difficult to identify. Serum creatinine at birth reflects maternal serum levels. Levels decline in the first few weeks, making ‘baseline’ creatinine dynamic. While sensitive, urine output is difficult to measure and is often preserved despite AKI due to tubular immaturity. The RIFLE and KDIGO definitions were adapted for neonates but require validation (Supplementary Table S1). In practice, an increase in creatinine by ≥ 0.3 mg/dL detects AKI better than a 50% rise in creatinine. A peak creatinine value of ≥ 2.5 mg/dL (~eGFR < 10 mL/min/1.73 m2) indicates severe AKI, and values ≥ 0.5 mg/dL at discharge are of concern [4].
Epidemiology
In a prospective report, AKI affected ~5% of hospitalized patients and ~30% of patients in the pediatric intensive care unit (PICU) [5]. Three multicenter studies inform on the global epidemiology of pediatric AKI. Using KDIGO criteria, the Assessment of Worldwide Acute Kidney Injury, Renal Angina, and Epidemiology (AWARE) study reported AKI in 26.9% of critically ill patients [6]. The Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study reported AKI in 29.9% newborns, most commonly in those born at < 29 wk of gestation [7]. In the 0by25 Global Snapshot study, similar proportions of hospitalized patients had community-acquired and incident AKI [8]. Patients with AKI in low- and middle-income countries were older than their counterparts in high-income countries, usually had community-acquired AKI, and commonly had severe AKI [8].
Etiology and Risk Factors
Based on pathophysiology, AKI is categorized as prerenal or functional, renal or intrinsic, and postrenal or obstructive. The Acute Dialysis Quality Initiative network summarized the risk factors into environmental, socioeconomic/cultural, process of care, acute exposures, and inherent factors [9]. Risk factors, etiology, and outcomes of AKI differ between resource-limited and resource-sufficient regions and for community-acquired and hospital-acquired AKI. AKI in developing countries is often community-acquired, caused by diarrheal dehydration, infections (sepsis, malaria, dengue, scrub typhus, leptospirosis), hemolytic uremic syndrome, glomerulonephritis, snake envenomation, and toxic medications [5, 10, 11]. In contrast, hospital-acquired AKI is more common in the developed world and is often associated with major surgery, septicemia, and/or multiorgan failure [11].
AKI in neonates is most often prerenal and is caused by systemic hypoperfusion due to placental hypoperfusion, birth asphyxia, insensible losses (e.g., preterm neonates on phototherapy), diarrheal dehydration, third-space losses (sepsis, necrotizing enterocolitis), congenital heart disease, post-cardiac surgery, and following nephrotoxic therapies [7].
Evaluation
Investigations in critically ill patients with AKI are directed at identifying the etiology of AKI and screening for complications (Table 2). Urinary indices to discriminate functional from intrinsic AKI, e.g., urine sodium (</≥ 20 mEq/L), fractional excretion of sodium (< 1%/≥ 1%), fractional excretion of urea (< 35%/> 50%), osmolality (> 400/< 350 mOsm/kg) and specific gravity (> 1020/< 1012), have limited utility in patients receiving diuretics and/or IV fluids. A kidney biopsy is indicated when suspecting glomerulonephritis or interstitial nephritis with unexplained AKI and allograft dysfunction. Various tools have been used to identify critically ill patients without AKI or with mild AKI who are at risk of severe AKI, the need for KRT, and mortality (Table 3).
Fluid Overload
Fluid overload may not just indicate but also lead to AKI by affecting multiple organs, e.g., increased intra-abdominal pressure compromises gas exchange, myocardial performance, intestinal function, and renal perfusion, and delayed wound healing promotes inflammation. Meta-analyses of observational studies indicate that the degree of fluid overload correlates with mortality, with a 19% increase in risk for every liter of fluid overload (34 studies, 31,076 adults) and 6% for every percentage point increment (44 studies, 7507 children) [12, 13]. Fluid overload was associated with an increased risk of mechanical ventilation and AKI in critically ill children [13] and neonates (17 studies, 4772 neonates) [14]. While a threshold of > 10% defines ‘clinically significant’ fluid overload that merits KRT, lower values may also impact outcomes.
Renal Angina Index
The renal angina index (RAI), a product of the risk and injury scores assessed 8–12 h after ICU admission, has emerged as a satisfactory bedside tool to predict the occurrence of severe AKI in patients admitted to PICU (Table 3). A meta-analysis (22 studies, 14,001 children) found that RAI > 8 predicts day 3 severe AKI with 86% sensitivity and 77% specificity (AUC 0.88), and the need for KRT with 82% sensitivity and 74% specificity (AUC 0.85) [15].
Furosemide Stress Test (FST)
In early AKI, the response to furosemide might indicate tubular integrity. It was first described in critically ill adults, in whom a urine output of < 200 mL over 2 h following 1 mg/kg IV furosemide satisfactorily predicted stage-3 AKI (87.1% sensitivity, 84.1% specificity, and AUC 0.87) [16]. Subsequent studies confirm that FST outperforms conventional biomarkers in predicting progression to stage-3 AKI, the need for KRT, and mortality, allowing decisions on postponing KRT until conventionally indicated [17]. A meta-analysis (11 studies, 1366 critically ill adults) found that FST predicts AKI progression and the need for KRT with respective sensitivity and specificity of 81% vs. 84% and 88% vs. 77% [18]. However, FST is not validated in children. In three retrospective studies, urine output following IV furosemide predicted severe AKI in infants after cardiac surgery [19,20,21]. In a prospective study on 51 critically ill children with AKI stages 1–2, using a threshold of > 2 mL/kg urine over 2 h after furosemide administration, FST outperformed plasma NGAL and proenkephalin in predicting severe AKI and the need for KRT [22]. Larger prospective pediatric studies are necessary before FST is applied bedside.
Biomarkers
Despite decades of research, a troponin-like diagnostic and predictive biomarker for pediatric AKI remains elusive. NGAL remains the most widely tested biomarker in children. In a meta-analysis of 56 studies investigating 49 biomarkers in 8617 children post-cardiac surgery, the urine NGAL to creatinine ratio had the highest diagnostic odds ratio (91%) with 91.3% sensitivity and 89.7% specificity [23], and combining it with RAI improved the diagnostic accuracy for severe AKI [15].
Dynamic Risk Prediction and Combination Tools
Dynamic models of AKI risk prediction that improved AKI recognition based on electronic inpatient records in individual studies include alerts for nephrotoxin exposure (e.g., NINJA), AKI care bundles [24], the four-pronged FOKIS (correlates with mortality; Table 3) [25], and a neonatal risk score (STARZ; predicts AKI with 82% sensitivity and 91.7% specificity) [26]. Experts recommend that clinical assessment and investigations be combined with biochemical and functional biomarkers to stratify the risk of severe AKI and inform decisions on its prevention and management [27].
Management
This section discusses important concerns in the nondialytic management of AKI, excluding the management of complications and specific etiologies of AKI.
Fluid Management
Assessment of volume status is critical in all patients with AKI. While hypovolemia, e.g., following diarrheal dehydration, is detrimental, fluid overload is associated with adverse outcomes. The quantity, rate, and type of fluids to be used in critically ill patients is debated. Since hyperchloremia is associated with tubular injury, delayed recovery of kidney function, and increased mortality, balanced/buffered crystalloids with lower chloride concentrations have been preferred, although evidence is awaited [28]. The use of hydroxylethyl starch or IV albumin is not recommended [28]. Fluid stewardship, following ‘ROSE’ (resuscitation, optimization, stabilization, and evaluation/deresuscitation) for fluid therapy in septic shock, and attention to the four D’s (drug, duration, dosing, and de-escalation) is suggested [29].
Diuretics and Adjunctive Therapies
A meta-analysis (28 trials, 3228 adults with or at risk for AKI) showed that intermittent IV furosemide did not influence rates of AKI, need for KRT, or death [30]. Continuous infusion of low-dose furosemide in early AKI did not alter the progression of AKI or to KRT in adults or children [31, 32]. In the absence of clear benefit, diuretics should not be used to prevent and/or treat AKI, and KRT should be initiated when indicated [33].
There is insufficient evidence to support the use of mannitol, dopamine, fenoldopam, atrial natriuretic peptide, nesiritide, or rasburicase in preventing AKI or the need for KRT [33]. The only evidence-based intervention to prevent AKI is theophylline (single IV dose of 5–8 mg/kg within an hour of birth) in neonates with severe perinatal asphyxia [33].
Drug Dosing and Nephrotoxic Medications
Drug dosing is difficult in critically ill patients with AKI due to altered pharmacokinetics, reduced clearances, extracorporeal removal during KRT, and dynamic organ functions. Evidence to guide dosing for most drugs is limited, especially for children. Antibiotic dosing while on KRT follows recommendations for adults [34]. For other medications, dosing while on continuous KRT (CKRT) assumes either a clearance of ~20–50 mL/min/1.73 m2 or is estimated from the CKRT effluent rate [35]. Where feasible, therapeutic drug monitoring should support decisions regarding dosing. Electronic alerts (‘NINJA’, ‘Baby NINJA’) following use of nephrotoxic medications (Supplementary Table S2) reduce the incidence of drug-induced AKI [36].
Nutrition
Almost one-third of critically ill patients are severely malnourished; prevailing nutritional practices meet two-thirds of energy and one-third of protein requirements, fluid restriction accentuates underfeeding, and nutrient clearances are increased in CKRT [37]. Achieving nutritional goals enterally versus parenterally was linked to improved survival, while high protein intake does not retard renal recovery [38]. According to international guidelines, enteral nutrition is preferred and should commence preferably within 2 d, targeting calories at 120%–130% of basal metabolic needs, and protein intake of 2–3 g/kg/d [39]. Frequent review of the nutritional prescription, reassessing anthropometry, and monitoring blood levels of urea nitrogen, electrolytes, glucose, and lipids help achieve the nutritional goals.
Kidney Replacement Therapy
Timing of KRT
KRT should be promptly initiated in patients with AKI presenting with features of fluid overload > 10%, pulmonary edema, uremia (urea > 200 mg/dL) with encephalopathy, persistent hyperkalemia (potassium > 6 mEq/L), metabolic acidosis (pH < 7.15), and refractory or symptomatic hyponatremia or severe hyperuricemia or hyperphosphatemia associated with tumor lysis syndrome (TLS). Similarly, initiating KRT promptly in patients with hyperammonemia/metabolic decompensation in cases of inborn errors of metabolism (e.g., propionic acidemia, methylmalonic acidemia), or poisoning and intoxications (e.g., lithium, salicylate, valproic acid, metformin) improves outcomes.
The timing of initiation of KRT, in the absence of the above-mentioned factors, is debatable. Five recent RCTs in adult patients have reported the risks and benefits of early versus late initiation of KRT, and a meta-analysis (10 trials, 2143 adults) failed to find any benefit from initiating KRT early [40]. Rates of dialysis dependence and treatment-related adverse events were higher in patients initiated on KRT early. However, waiting until KRT was indicated urgently, was associated with higher mortality in AKIKI-2. These results cannot be extrapolated to pediatric patients, in whom retrospective studies have associated delayed initiation of KRT with adverse outcomes [41, 42]. From a practical perspective, it seems prudent to initiate KRT if oliguria and azotemia persist beyond 48–72 h, even in the absence of clinical or metabolic complications of AKI.
Modalities of KRT
The advantages and disadvantages of different modalities of KRT are summarized in Table 4 and discussed below. The choice of modality in critically ill children is guided more by patient size, hemodynamic stability, the feasibility of securing vascular access and appropriately sized dialysis catheters, and available technical expertise for extracorporeal therapies, than dialysis efficiency [43]. Hence, intermittent hemodialysis is preferred in hemodynamically stable older children, and peritoneal dialysis is favored in young infants in whom vascular access, dialyzer size, and the nonavailability of suitable machines preclude hemodialysis. In hemodynamically unstable patients, prolonged intermittent KRT is preferred over PD, which is inefficient in the presence of mesenteric ischemia.
Peritoneal Dialysis
Peritoneal dialysis (PD) is a simple and inexpensive modality of KRT that is feasible in low- resource settings, infants, and hemodynamically unstable patients. PD is relatively contraindicated in patients with recent abdominal surgery, peritonitis, or an inguinal hernia. PD is less preferred when rapid clearance is required, e.g., in severe hyperammonemia. The International Society of Peritoneal Dialysis (ISPD) recently provided optimal and minimum standards for peritoneal access, dialysate fluids, and PD prescription for children with AKI [44]. A soft (Tenckhoff) catheter, inserted surgically or bedside using the Seldinger technique, is preferred over rigid (stiff) catheters and improvisations (nasogastric tube, intercostal drain) [44]. Measures to avoid peritonitis include administering an antibiotic dose 30 min before catheter insertion, removing rigid catheters within 72 h, preferring automated cyclers over manually operated open systems, and using closed dialysate circuits for manual cycles (Y-connections in older children and ready-made closed circuits with buretrols in infants). Commercially prepared dialysates are preferred to locally prepared fluids [44]. Higher than usual dextrose concentrations (1.5%–1.7%) are used if the ultrafiltration target is not met. Bicarbonate-based solutions are preferred to lactate-containing solutions in patients with hepatic dysfunction, hemodynamic instability, and worsening metabolic acidosis. The dwell volume should be 30–40 mL/kg (800–1100 mL/m2), except if initiating PD soon after surgical catheter placement, when short (10–15 mL/kg) dwells are preferred to avoid leakage. The dwell duration depends on ultrafiltration and solute removal targets; e.g., it is 20–30 min in the presence of hyperkalemia and acidosis and lengthened to 2–3 h once azotemia improves. Patients are monitored closely for dehydration, hypervolemia or sepsis, and for electrolytes, pH and glucose every 8–12 h.
Continuous-flow peritoneal dialysis is considered when conventional PD is ineffective, particularly when dwell volume is limited by post-surgery status or high ventilatory settings. The prescription comprises low dwell volumes (10–20 mL/kg, high dialysate flow (50–100 mL/min per 1.73 m2), and slow ultrafiltration (2.5 mL/min per 1.73 m2) [44].
Intermittent Hemodialysis
Hemodialysis enables rapid and efficient solute clearance and ultrafiltration, making it the most suited modality for hemodynamically stable patients with vascular access, particularly in urgent settings, e.g., poisoning, TLS, hyperammonemia, pulmonary edema. Therapy is intermittent (3–4 h daily), allowing downtime for diagnostic procedures. However, hemodialysis requires technical expertise, especially with pediatric patients. Hemodialysis is difficult in young children in the absence of pediatric-specific equipment and large-bore vascular access, typically a double-lumen hemodialysis catheter in the internal jugular or femoral vein; subclavian access carries the risk of stenosis.
The hemodialysis prescription is similar in AKI and CKD and aimed at maximizing solute clearance and fluid removal without causing adverse events [45]. The prescription varies by the child’s size, including dialyzer size (0.7–1 × body surface area), tubings (neonatal, pediatric, or adult lines), and rates of blood flow (4–8 mL/kg/min), dialysate flow (1.5–2 × blood flow rate), and ultrafiltration (≤ 0.2 mL/kg/min or ≤ 5% weight). The dialysate composition may require alteration for severe hypernatremia or hyperkalemia. Session length and frequency are guided by the solute and ultrafiltration targets. If the extracorporeal blood volume, estimated by the dialyzer size and tubings, exceeds 10% of the blood volume, the circuit is primed with blood, 5% albumin, or saline to avoid intradialytic hypotension. Frequent assessment of dry weight, close monitoring of vital signs, and noninvasive blood volume monitoring enable safe ultrafiltration.
Continuous Kidney Replacement Therapy
CKRT is preferred in critically ill, hemodynamically unstable patients since it permits gradual and continuous solute clearance and ultrafiltration, thus avoiding rapid solute fluxes, which carry risks of intradialytic hypotension and raised intracranial pressure, while allowing liberal use of boluses, infusions and adequate nutritional support. However, CKRT is not uniformly available across PICUs due to the requirement of expensive equipment, consumables, and technical expertise.
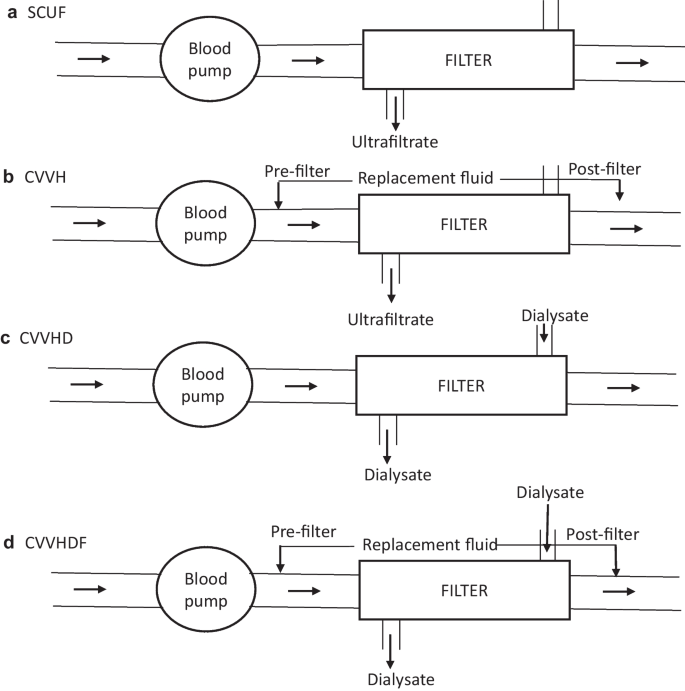
The principles of solute clearance and ultrafiltration in CKRT are similar to those of intermittent hemodialysis but include lower flow rates. The most commonly used modes include slow continuous ultrafiltration (SCUF), continuous venovenous hemofiltration (CVVH), continuous venovenous hemodialysis (CVVHD) and continuous venovenous hemodiafiltration (CVVHDF) (Fig. 1). Two modalities (CVVH and CVVHD) rely on replacement fluid to mediate predominantly convective clearance. The choice of modality is guided by therapy goals (ultrafiltration versus solute clearance), available circuits, and physician preference. While a conventional dose of KRT is a dialysis fluid rate of 2000 mL/1.73 m2/h, in settings such as hyperammonemia, higher doses (4000–8000 mL/1.73 m2/h) are preferred. Circuit priming averts the risk of hypotension with high extracorporeal volumes. Outcomes do not differ significantly among various modalities [46].
Circuit diagrams for the various modes of continuous kidney replacement therapy (CKRT). Blood flows from left to right from the patient to the blood pump and then to the filter, from which it is returned to the patient. a Slow continuous ultrafiltration (SCUF): In this modality, there is no diffusive clearance and only ultrafiltrate (UF) is generated across the filter; this method is preferred for isolated UF removal when kidney function is normal. b Continuous venovenous hemofiltration (CVVH): Replacement fluid is run either pre- or post-filter in a volume to replace the effluent; excess effluent is removed to ensure the UF desired for negative fluid balance; clearance is convective rather than diffusive. c Continuous venovenous hemodialysis (CVVHD): Blood flows across the filter in a countercurrent fashion with the dialysate fluid, and the effluent predominantly consists of dialysate fluid with minimal, if any, UF, as in intermittent hemodialysis. d Continuous venovenous hemodiafiltration (CVVHDF): This modality combines CVVH with CVVHD, such that the blood and dialysis fluid run in counter-current directions, the replacement fluid is either pre- or post-filter, and the effluent comprises the dialysate and replacement fluids
Prolonged Intermittent Therapies
Sustained low-efficiency dialysis (SLED) is a form of prolonged intermittent renal replacement therapy (PIRRT), combining the advantages of intermittent hemodialysis and CKRT [47]. SLED utilizes conventional hemodialysis machines, dialyzers, and tubings, but the blood and dialysate flow rates are lower (QB: 3–5 mL/kg/min; ≤ 150 mL/min in older children; QD: ≤ 2 times the QB; ≤ 300 mL/min in older children) and the sessions are longer (6–12 h). SLED achieves similar solute clearance and ultrafiltration as conventional hemodialysis without inducing hemodynamic instability in critically ill patients. The minimum dialysate rate is limited by the lack of pediatric-specific dialysis machines. Ultrafiltration goals (rate ≤ 0.2 mL/kg/min) and the need for anticoagulation and circuit priming are similar to hemodialysis. SLED may be performed daily or on alternate days at the physician’s discretion. The addition of convective clearance, as in hemodiafiltration (SLEDD-F) improves solute clearances.
Given its accessibility, lower cost, shorter session length and lesser technical complexity, SLED is increasingly preferred over CKRT in adult and pediatric critically ill patients. A meta-analysis (11 studies, 1160 critically ill adults) found that SLED and CKRT were associated with similar ultrafiltration and comparable lengths of hospital stay and rates of mortality, dialysis dependence, and renal recovery [48]. Based on experience with 203 children in three retrospective and two prospective series, SLED appears safe, effective, and feasible in critically ill pediatric patients [47, 49, 50].
Anticoagulation
Anticoagulation is necessary to prevent clotting in extracorporeal circuits, particularly with small catheters and low blood flow and/or high ultrafiltration rates. Anticoagulation is particularly challenging during CKRT, which requires prolonged circuit patency at slow blood flow rates. Unfractionated heparin is used most commonly and is injected into the circuit as an IV bolus, followed by its infusion or intermittent boluses [51]. In patients with coagulopathy, intermittent flushes with saline can be used to keep the circuit patent during intermittent hemodialysis and short SLED sessions; however, this limits ultrafiltration. Citrate-based protocols, allowing regional anticoagulation limited to the circuit rather than systemic, are particularly useful in patients with coagulopathy on CKRT. A meta-analysis on anticoagulation indicates that regional citrate anticoagulation provides better circuit and filter life than heparin [51]. However, careful titration is essential to prevent hypocalcemia, and dose adjustments are required in the presence of hepatic dysfunction. Less established options of anticoagulation include low molecular weight heparin, prostacyclin, direct thrombin inhibitors (argatroban), fondaparinux, and nafamostat [51].
Extracorporeal Therapy in Infants
Delivering KRT to infants is challenging since conventional hemodialysis equipment is designed for adults, with extracorporeal volumes and calibration errors that are unacceptably high for children < 17 kg body weight. Three machines, namely the Cardiorenal Pediatric Emergency Dialysis Machine (CARPEDIEM®), Newcastle Infant Dialysis and Ultrafiltration System (NIDUS®), and Aquadex®, can perform KRT in neonates and young infants using low extracorporeal blood volume and a slow blood flow rate while permitting precise calibration of ultrafiltration. Multicenter retrospective data confirm their safety and feasibility in infants with AKI [52, 53]. However, these machines are not marketed in India, and most centers use conventional machines, e.g., Prismaflex®, to deliver CKRT to infants.
Specific Settings
AKI affects 36.2% (95% CI 18.8–55.8) patients with acute or acute-on chronic liver failure, chiefly as functional AKI or acute tubular necrosis following gastrointestinal bleeding, vomiting, and hepatorenal syndrome [54]. Volume expansion with fluids and albumin, loop diuretics, and vasoconstrictors (vasopressin, octreotide) are useful in hepatorenal syndrome. Beyond conventional indications, KRT is considered in the presence of hyperammonemia and hepatic encephalopathy. CKRT is preferred to intermittent hemodialysis since it permits the slow, continuous removal of ammonia, thus reducing the risk of cerebral edema [54]. Anticoagulation is avoided in the presence of coagulopathy. In the absence of conventional extracorporeal liver support (molecular adsorbent recirculating system, Prometheus, single-pass albumin dialysis) for fulminant acute liver failure, therapeutic plasma exchanges might serve as a bridge to liver transplantation [55].
During acute metabolic crises in inborn errors of metabolism, toxic metabolites like ammonia and leucine accumulate rapidly, leading to cerebral edema and neurological sequelae. CKRT has an effective modality to remove these metabolites and is initiated in patients with (i) rapidly deteriorating neurological status and blood ammonia > 150 μmol/L; (ii) moderate or severe encephalopathy; or (iii) persistently high ammonia levels > 400 μmol/L; (iv) rapid rise to > 300 μmol/L, despite adequate medical measures (protein restriction; nitrogen scavengers, e.g., sodium benzoate) [56]. CVVHD is recommended, with blood and dialysate flow rates of 30–50 mL/min and ≥ 2500–3000 mL/1.73 m2/h, respectively, and a dialysate to blood flow rate ratio of > 1.5 [56]. While intermittent hemodialysis also clears metabolites rapidly, it carries the risks of post-session rebound and intradialytic hypotension. Peritoneal dialysis is less effective and unreliable in clearing large metabolites (e.g., organic acids) [56].
AKI is an important component of the Cairo–Bishop criteria used to define and classify TLS. Risk stratification, adequate hydration, and routine and early use of allopurinol and/or rasburicase have reduced its incidence. The threshold for KRT initiation is low; chief indications include refractory hyperkalemia, hyperphosphatemia, hyperuricemia, metabolic acidosis, and fluid overload unresponsive to diuretics [57].
Mechanisms for AKI in COVID-19 include hypoxia, hypotension, inflammation associated with a cytokine storm, and exposure to nephrotoxic agents. About 10% of children hospitalized with COVID-19 developed AKI, of which the majority were mild and uncommonly merited KRT [58]. The PCRRT workgroup has outlined key considerations when managing critically ill children with COVID-19 and AKI [59].
Outcomes
Recent multicenter studies show that AKI in critically ill children is independently associated with prolonged ICU or hospital stay and high mortality, with stepwise increments by AKI stage [6,7,8]. Mortality is higher in developing countries, correlating inversely with per capita income and health expenditure [1, 8]. In a systematic review of five studies on severe AKI requiring KRT in children, the cumulative incidence (95% CI) per 100 patient-years of reduced GFR (< 90 mL/min/1.73 m2), proteinuria, hypertension and kidney failure were 6.3 (5.1–7.5), 3.1 (2.1–4.1), 1.4 (0.9–2.1), and 0.9 (0.6–1.4), respectively, underscoring the importance of prolonged follow up for development of CKD in patients with AKI [60].
References
Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138.
Xu X, Nie S, Zhang A, et al. A new criterion for pediatric AKI based on the reference change value of serum creatinine. J Am Soc Nephrol. 2018;29:2432–42.
Askenazi D, Abitbol C, Boohaker L, et al. Neonatal kidney collaborative. Optimizing the AKI definition during first postnatal week using Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) cohort. Pediatr Res. 2019;85:329–338.
Mehta P, Sinha A, Sami A, et al. Incidence of acute kidney injury in hospitalized children. Indian Pediatr. 2012;49:537–42.
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. AWARE Investigators. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20.
Jetton JG, Boohaker LJ, Sethi SK, et al. Neonatal Kidney Collaborative (NKC). Incidence and outcomes of neonatal acute kidney injury (AWAKEN): A multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1:184–94.
Macedo E, Cerdá J, Hingorani S, et al. Recognition and management of acute kidney injury in children: The ISN 0by25 Global Snapshot Study. PLoS ONE. 2018;13:e0196586.
Kashani K, Macedo E, Burdmann EA, et al. Acute kidney injury risk assessment: Differences and similarities between resource-limited and resource-rich countries. Kidney Int Rep. 2017;2:519–29.
Krishnamurthy S, Mondal N, Narayanan P, Biswal N, Srinivasan S, Soundravally R. Incidence and etiology of acute kidney injury in southern India. Indian J Pediatr. 2013;80:183–9.
Cerdá J, Bagga A, Kher V, Chakravarthi RM. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol. 2008;4:138–53.
Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid overload and mortality in adult critical care patients-A systematic review and meta-analysis of observational studies. Crit Care Med. 2020;48:1862–70.
Alobaidi R, Morgan C, Basu RK, et al. Association between fluid balance and outcomes in critically ill children: A systematic review and meta-analysis. JAMA Pediatr. 2018;172:257–68.
Matsushita FY, Krebs VLJ, de Carvalho WB. Association between fluid overload and mortality in newborns: A systematic review and meta-analysis. Pediatr Nephrol. 2022;37:983–92.
Meena J, Kumar J, Thomas CC, et al. Diagnostic accuracy of renal angina index alone or in combination with biomarkers for predicting acute kidney injury in children. Pediatr Nephrol. 2022;37:1263–75.
Chawla LS, Davison DL, Brasha-Mitchell E, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17:R207.
McMahon BA, Chawla LS. The furosemide stress test: Current use and future potential. Ren Fail. 2021;43:830–9.
Chen JJ, Chang CH, Huang YT, Kuo G. Furosemide stress test as a predictive marker of acute kidney injury progression or renal replacement therapy: A systemic review and meta-analysis. Crit Care. 2020;24:202.
Kakajiwala A, Kim JY, Hughes JZ, et al. Lack of furosemide responsiveness predicts acute kidney injury in infants after cardiac surgery. Ann Thorac Surg. 2017;104:1388–94.
Penk J, Gist KM, Wald EL, et al. Furosemide response predicts acute kidney injury in children after cardiac surgery. J Thorac Cardiovasc Surg. 2019;157:2444–51.
Borasino S, Wall KM, Crawford JH, et al. Furosemide response predicts acute kidney injury after cardiac surgery in infants and neonates. Pediatr Crit Care Med. 2018;19:310–7.
Krishnasamy S, Sinha A, Bagga A, Hari P. The diagnostic performance of furosemide stress test in predicting progression to acute kidney injury stage 3: Need for kidney replacement therapy and mortality. The 34th Annual Conference of Indian Society of Pediatric Nephrology selected abstracts. Asian J Pediatr Nephrol. 2022;5:3–4.
Van den Eynde J, Schuermans A, Verbakel JY, et al. Biomarkers of acute kidney injury after pediatric cardiac surgery: A meta-analysis of diagnostic test accuracy. Eur J Pediatr. 2022;181:1909–21.
Kotwal S, Herath S, Erlich J, et al. Electronic alerts and a care bundle for acute kidney injury - An Australian cohort study. Nephrol Dial Transplant. 2022:gfac155. https://doi.org/10.1093/ndt/gfac155.
Akcan-Arikan A, Gebhard D, Arnold MA, Loftis LL, Kennedy CE. Fluid overload and kidney injury score (FOKIS): A multidimensional real-time assessment of renal disease burden in the critically ill patient. Pediatr Crit Care Med. 2017;18:524–30.
Wazir S, Sethi SK, Agarwal G, et al. Neonatal acute kidney injury risk stratification score: STARZ study. Pediatr Res. 2022;91:1141–8.
Ostermann M, Zarbock A, Goldstein S, et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: A consensus statement. JAMA Netw Open. 2020;3:e2019209.
Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21:e52-106.
Malbrain MLNG, Van Regenmortel N, Saugel B, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018;8:66.
Bove T, Belletti A, Putzu A, et al. Intermittent furosemide administration in patients with or at risk for acute kidney injury: Meta-analysis of randomized trials. PLoS ONE. 2018;13:e0196088.
Bagshaw SM, Gibney RTN, Kruger P, Hassan I, McAlister FA, Bellomo R. The effect of low-dose furosemide in critically ill patients with early acute kidney injury: A pilot randomized blinded controlled trial (the SPARK study). J Crit Care. 2017;42:138–46.
Abraham S, Rameshkumar R, Chidambaram M, et al. Trial of furosemide to prevent acute kidney injury in critically ill children: A double-blind, randomized, controlled trial. Indian J Pediatr. 2021;88:1099–106.
Kellum JA, Lameire N. KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care. 2013;17:204.
Hoff BM, Maker JH, Dager WE, Heintz BH. Antibiotic dosing for critically ill adult patients receiving intermittent hemodialysis, prolonged intermittent renal replacement therapy, and continuous renal replacement therapy: An update. Ann Pharmacother. 2020;54:43–55.
Schetz M. Drug dosing in continuous renal replacement therapy: General rules. Curr Opin Crit Care. 2007;13:645–51.
Goldstein SL, Mottes T, Simpson K, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 2016;90:212–21.
Kyle UG, Akcan-Arikan A, Orellana RA, Coss-Bu JA. Nutrition support among critically ill children with AKI. Clin J Am Soc Nephrol. 2013;8:568–74.
Kyle UG, Akcan-Arikan A, Silva JC, Goldsworthy M, Shekerdemian LS, Coss-Bu JA. Protein feeding in pediatric acute kidney injury is not associated with a delay in renal recovery. J Ren Nutr. 2017;27:8–15.
Sethi SK, Maxvold N, Bunchman T, Jha P, Kher V, Raina R. Nutritional management in the critically ill child with acute kidney injury: A review. Pediatr Nephrol. 2017;32:589–601.
Gaudry S, Hajage D, Benichou N, et al. Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: A systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 2020;395:1506–15.
Cortina G, McRae R, Hoq M, et al. Mortality of critically ill children requiring continuous renal replacement therapy: effect of fluid overload, underlying disease, and timing of initiation. Pediatr Crit Care Med. 2019;20:314–22.
Modem V, Thompson M, Gollhofer D, Dhar AV, Quigley R. Timing of continuous renal replacement therapy and mortality in critically ill children*. Crit Care Med. 2014;42:943–53.
Ye Z, Wang Y, Ge L, et al. Comparing renal replacement therapy modalities in critically ill patients with acute kidney injury: A systematic review and network meta-analysis. Crit Care Explor. 2021;3:e0399.
Nourse P, Cullis B, Finkelstein F, et al. ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 Update (paediatrics). Perit Dial Int. 2021;41:139–57.
Fischbach M, Edefonti A, Schroder C, Watson A. European Pediatric Dialysis Working Group. Hemodialysis in children: General practical guidelines. Pediatr Nephrol. 2005;20:1054–66.
Zha J, Li C, Cheng G, Huang L, Bai Z, Fang C. The efficacy of renal replacement therapy strategies for septic-acute kidney injury: A PRISMA-compliant network meta-analysis. Medicine (Baltimore). 2019;98:e15257.
Sethi SK, Mittal A, Nair N, et al. Pediatric Continuous Renal Replacement Therapy (PCRRT) expert committee recommendation on prescribing prolonged intermittent renal replacement therapy (PIRRT) in critically ill children. Hemodial Int. 2020;24:237–51.
Dalbhi SA, Alorf R, Alotaibi M, et al. Sustained low efficiency dialysis is non-inferior to continuous renal replacement therapy in critically ill patients with acute kidney injury: A comparative meta-analysis. Medicine (Baltimore). 2021;100:e28118.
Yadav M, Tiwari AN, Lodha R, et al. Feasibility and efficacy of sustained low-efficiency dialysis in critically ill children with severe acute kidney injury. Indian J Pediatr. 2022. https://doi.org/10.1007/s12098-022-04214-z.
Shiri S, Naik NM, Av L, Vasudevan A. Sustained low efficiency dialysis in critically ill children with acute kidney injury: Single-center observational cohort in a resource-limited setting. Pediatr Crit Care Med. 2022. https://doi.org/10.1097/PCC.0000000000003127.
Raina R, Agrawal N, Kusumi K, Pandey A, Tibrewal A, Botsch A. A meta-analysis of extracorporeal anticoagulants in pediatric continuous kidney replacement therapy. J Intensive Care Med. 2022;37:577–94.
Goldstein SL, Vidal E, Ricci Z, et al. Survival of infants treated with CKRT: comparing adapted adult platforms with the Carpediem™. Pediatr Nephrol. 2022;37:667–75.
Menon S, Broderick J, Munshi R, et al. Kidney support in children using an ultrafiltration device: A multicenter, retrospective study. Clin J Am Soc Nephrol. 2019;14:1432–40.
Raina R, Sethi SK, Filler G, et al. PCRRT Expert Committee ICONIC position paper on prescribing kidney replacement therapy in critically sick children with acute liver failure. Front Pediatr. 2022;9:833205.
Alexander EC, Deep A. Therapeutic plasma exchange in children with acute liver failure (ALF): is it time for incorporation into the ALF armamentarium? Pediatr Nephrol. 2022;37:1775–88.
Raina R, Bedoyan JK, Lichter-Konecki U, et al. Consensus guidelines for management of hyperammonaemia in paediatric patients receiving continuous kidney replacement therapy. Nat Rev Nephrol. 2020;16:471–82.
Matuszkiewicz-Rowinska J, Malyszko J. Prevention and treatment of tumor lysis syndrome in the era of onco-nephrology progress. Kidney Blood Press Res. 2020;45:645–60.
Raina R, Mawby I, Chakraborty R, et al. Acute kidney injury in COVID-19 pediatric patients in North America: Analysis of the virtual pediatric systems data. PLoS ONE. 2022;17:e0266737.
Raina R, Chakraborty R, Sethi SK, Bunchman T. Kidney replacement therapy in COVID-19 induced kidney failure and septic shock: A Pediatric Continuous Renal Replacement Therapy [PCRRT] position on emergency preparedness with resource allocation. Front Pediatr. 2020;8:413.
Greenberg JH, Coca S, Parikh CR. Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC Nephrol. 2014;15:184.
Acknowledgements
The authors acknowledge the support of the Indian Council of Medical Research (5/7/1090/2013-RHN).
Author information
Authors and Affiliations
Contributions
SK made substantial contribution to the acquisition, analysis, and interpretation of data and drafted the manuscript; AS, SK, and AB made substantial contributions to the draft of the manuscript, revised it critically, and approved the final manuscript for submission; AB agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of the work are appropriately investigated and resolved. AB will act as the guarantor for this paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krishnasamy, S., Sinha, A. & Bagga, A. Management of Acute Kidney Injury in Critically Ill Children. Indian J Pediatr 90, 481–491 (2023). https://doi.org/10.1007/s12098-023-04483-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-023-04483-2