Abstract
Objective
To evaluate the factors associated with mortality of a multicentric cohort of hospitalized COVID-19 patients, 0–18 y old, from 42 centers across India.
Methods
The National Clinical Registry for COVID-19 (NCRC) is an on-going prospective data collection platform enrolling COVID-19 patients diagnosed by real-time PCR or rapid antigen test. The data are collected in prestructured e-capture forms. The sociodemographic, clinical, laboratory, and hospital outcome data from 1st September 2020 to 20th February 2022 were analyzed.
Results
Of the 1244 enrolled hospitalized COVID-19 patients aged 0–18 y, 98 and 124 were infants and neonates, respectively. Only 68.6% children were symptomatic at admission, with fever being the most common symptom. Diarrhea, rash, and neurological symptoms were also noted. At least 1 comorbidity was present in 260 (21%) children. The in-hospital mortality rate was 6.2% (n = 67), the highest in infants (12.5%). Altered sensorium (aOR: 6.8, CI: 1.9, 24.6), WHO ordinal scale ≥ 4 at admission (aOR: 19.6, CI: 8.0, 47.8), and malignancy (aOR: 8.9, 95% CI: 2.4, 32.3) were associated with higher odds of death. Malnutrition did not affect the outcome. Mortality rates were similar across the three waves of the pandemic, though a significant shift towards the under-five group was observed in the third wave.
Conclusion
This multicentric cohort of admitted Indian children showed that the COVID-19 was milder in children than adults, and the pattern was consistent across all waves of the pandemic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The impact of the COVID-19 pandemic has been widespread, and no one has been spared. COVID-19 severely affected the geriatric population and adults with comorbidity; children have also been affected and have suffered from the pandemic [1, 2]. Children are a particularly vulnerable group, especially the younger ones, considering their limited ability to express and take care of themselves [3].
Since January 2020, many case series as well as retrospective analyses describing the clinical features and course of COVID-19 in Indian children have been published. However, the majority of these studies recruited a limited number of children, and described the course during the first or second wave of the pandemic [4,5,6,7,8,9]. Data regarding the especially vulnerable population of neonates and infants are scarce [10, 11]. Factors, modifiable or not, associated with COVID-related mortality have not been well studied in the pediatric population. In the current analysis, the authors present the details of hospitalized COVID-19 patients belonging to the age group 0–18 y from 42 government and private hospitals across India, spanning all three waves of the pandemic experienced in the country. They have also identified the factors associated with in-hospital mortality in pediatric COVID-19 patients.
Material and Methods
The National Clinical Registry for COVID-19 (NCRC) serves as a platform for on-going prospective data collection, which has been developed and maintained by the Indian Council of Medical Research (ICMR) in collaboration with the Ministry of Health & Family Welfare (MoHFW), Government of India, the All India Institute of Medical Sciences, New Delhi (AIIMS), and the ICMR-National Institute of Medical Statistics (ICMR-NIMS). The structure of the registry is available in the public domain (https://www.icmr.gov.in/tab1ar1.html). The details of the functioning of NCRC have been published elsewhere [12]. Across the network of the NCRC, participating hospitals recruited consecutive in-patients who had SARS-CoV-2 infection confirmed by real-time polymerase chain reaction (RT-PCR), nucleic acid amplification test (NAAT), or rapid antigen test (RAT). Data pertaining to participants 0–18 y of age were retrieved for the purpose of this record-based analysis.
Sociodemographic, clinical, laboratory, and hospital outcome data were analyzed. Categorical data have been presented as frequency and proportions, and continuous data with mean ± standard deviation or median (interquartile range), as appropriate. The logistic-regression model was used to identify factors associated with death among children. The death of a COVID-19 pediatric patient due to any cause during hospital stay was considered the outcome of interest for these analyses. Patients who were transferred to another hospital or left against medical advice (LAMA) were excluded from the outcome analyses, though their baseline characteristics were analyzed. Age, gender, anthropometric indices, pre-existing comorbidities, breathing difficulty, and central nervous system symptoms at admission as well as severity assessment by the WHO ordinal scale were used as explanatory variables in univariate analyses [13]. The variables with significant associations (p < 0.05) and those with known clinical or contextual importance were included in the multivariate logistic-regression model. Data analysis was carried out using STATA v14 (College Station, TX, US). The anthropometric z scores were calculated using the Anthro and Anthroplus software (WHO, Geneva).
Necessary approvals for the current investigation were obtained from the Central Ethics Committee for Human Research at ICMR (CECHR) as well as from the respective institutional ethics committees of each of the participating centers. Considering the observational nature of the registry, and the usage of anonymized data from the routine case records of the patients, a waiver of consent was granted by the aforementioned ethics committees.
Results
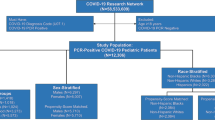
From 1st September 2020 to 20th February 2022, 1244 hospitalized COVID-19 patients aged 0–18 y were enrolled in the NCRC from 42 centers across India (Supplementary Fig. S1). While almost half of the patients belonged to the age group of 10–18 y, around one-fifth of the patients were infants and neonates; the youngest being 1-d old. Approximately 2/3rd of the patients in the 0–5 y age bracket had weight-for-age and weight-for-height z scores between −2 and +2. Weight for height also followed a similar pattern. Body mass index (BMI) was calculated among children of 6–18 y age. BMI of 77% children was between −2 and +2 standard deviations. A history of contact with a COVID-positive patient could be elicited in 22.1% (275/1244) of participants. The baseline demographic characteristics at admission are detailed in Table 1. Seventy-six percent of the children were admitted at a WHO ordinal scale of 3 (Table 1).
Only 68.6% of the children were symptomatic at admission. Among the symptomatic patients, fever was the most commonly reported symptom for all age groups. In children ≥ 5 y of age, fever was followed by a dry cough (33.3%) and breathing difficulty (22.3%). A substantial proportion of younger children between 1 and 5 y of age manifested gastrointestinal symptoms like vomiting (22.5%), diarrhea (17.7%), and abdominal pain (5.4%). Runny nose and wet cough were also reported in over 10% of children in this age group. Infants presented with fever, cough, and breathing difficulty, as well as gastrointestinal symptoms like vomiting (19.4%) and diarrhea (16.4%). Neurological symptoms such as altered consciousness (1.9%) and seizures (7.6%) were also observed in some infants. Neonates were largely asymptomatic (60.4%). The symptomatic neonates had fever, cough, breathing difficulty, and vomiting as their most common symptoms. Table 2 shows the symptom profile among symptomatic patients at baseline, segregated by age groups.
Out of the 1244 admitted children, 260 (21%) had at least one comorbidity. The most common comorbidity among children over 1 y of age was hematologic disorders and malignancy, while cardiac disease was more common among infants. Neonates did not present with comorbidities. Supplementary Table S1 shows the frequency and proportion of comorbidities across the various age groups.
Table 3 shows the laboratory profile of patients at admission. The laboratory markers were largely normal, except for the raised inflammatory markers. Figure 1 shows the treatment profile of the enrolled patients. The most commonly prescribed drug was azithromycin [n = 956/1244 (23.2%)], followed by steroids [n = 190/1244 (15.3%)], doxycycline [n = 175/1244 (14.1%)], ivermectin [n = 171/1244 (13.8%)], and anticoagulants [n = 111/1244 (8.9%)]. The rest of the drugs were administered to less than 5% of the patients. Oxygen supplementation was required by 296/1244 (23.8%) of patients, while noninvasive and mechanical ventilation were required by 51/1244 (4.1%) and 78/1244 (6.3%), respectively. The use of steroids was lowest among neonates (9.2%) with highest usage among the 10–18 y age group (17.8%). Oxygen requirement as well as invasive and noninvasive ventilation were highest among children less than 1 y of age (data not shown).
The data for outcome analysis were available for 1085 children. Of them, 93.8% (n = 1018) were discharged, and 6.2% (n = 67) died in the hospital. Children who were transferred to other hospitals (n = 127) or those who left against medical advice (n = 32) were excluded from the outcome analysis.
The proportion of deaths was highest in the age group 1 mo to 1 y [n = 15/120 (12.5%)], followed by neonates [n = 6/83 (7.2%)], 5–9 y [n = 11/173 (6.4%)], 10–18 y [n = 31/540 (5.7%)], and 1–4 y of children [n = 4/169 (2.4%)].
The median duration of hospital stay was 4 d (IQR: 2,9) and 6 d (IQR: 4,9) among survivors and nonsurvivors, respectively. Of the children who died, 50.8% had at least one comorbidity. Table 4 shows the proportion of outcomes across age categories, gender, pre-existing comorbidities, selected symptom categories, baseline clinical status, and nutritional status of the patients, along with unadjusted and adjusted odds ratios. The outcome was not significantly different across the age and gender categories. Bivariate logistic regression showed higher odds of occurrence of death among patients with chronic kidney disease (CKD), malignancy, and chronic neurological disease, shortness of breath/fast breathing at admission, altered sensorium or seizures at admission, or admission with the WHO ordinal scale 4 and above. On the multivariate model, the odds of occurrence of death were high among patients with malignancy (aOR: 8.9, 95% CI: 2.4, 32.3), altered sensorium or seizures at admission (aOR: 6.8, CI: 1.9, 24.6), and WHO ordinal scale 4 or above at admission (aOR: 19.6, CI: 8.0, 47.8), after adjusting for age, gender, other comorbidities, and symptoms. Anthropometric measures did not affect the occurrence of poor outcomes.
The patients were grouped based on the date of admission to the hospital for the three waves of the pandemic, with the first wave considered as the phase that lasted until 1st February 2021, the second wave being the one from 2nd February 2021 to 15th December 2022, and the third wave from 16th December to 20th February 2022. The number of children admitted during the first, second, and third waves were 422 (33.9%), 607 (48.8%), and 215 (17.3%), respectively. The proportion of patients admitted with comorbidities and mortality across the three waves was not significantly different, though a significant shift in age towards the under-5 group was observed at the time of the third wave (Supplementary Table S2).
Discussion
In this report, the authors have described the clinical presentation and course of COVID-19 illness in a large, representative cohort of 1244 pediatric patients admitted to 42 hospitals across India, which included 98 neonates and 142 infants. The majority of the participants were asymptomatic, and the mortality was lower compared to that observed in cohorts of adults.
About one-third (33.2%) participants in this cohort were asymptomatic, especially the neonates. Other Indian studies have also reported similar observations [6]. It seems that many of the pediatric admissions were either for monitoring or as an add-on diagnosis upon some other existing condition. Among symptomatic children, fever was the most common symptom across the age spectrum. This pattern mirrors that of the adult COVID-19 patients [12]. However, some interesting additional symptoms were observed in children, especially the younger ones. Gastrointestinal symptoms, including vomiting, diarrhea, and abdominal pain were seen in 18.76% of the under-5 s. Previously published systematic reviews have shown a varied frequency of gastrointestinal symptoms in the United Kingdom, the United States, and Wuhan [14]. The high expression of angiotensin-converting enzyme receptor 2 (ACE-2), and transmembrane protease serine 2 (TMPRSS2) in the gastric, duodenal, and rectal epithelium, and in the enterocytes of the ileum and colon are hypothesized to facilitate the entry of the virus in the gastrointestinal system [14]. Destruction of absorptive enterocytes by COVID-19 leads to an altered intestinal permeability, consequently leading to diarrhea [15]. Rash was another notable symptom in this age group. Neonates also presented with neurological abnormalities such as altered consciousness and convulsions. Other researchers have also reported neurological manifestations and complications [11].
In the present study, mortality in COVID-19 pediatric patient due to any cause during hospital stay was 6.2%. Such a figure is considerably lower than what is reported among hospitalized adult COVID-19 patients in India and abroad [12, 16, 17]. Other Indian reports on the pediatric population, mainly from tertiary care centers, have reported mortality ranging from 3.2% to 11.2% [6, 18, 19]. The present study, being a representative cohort, gives a holistic estimate of poor outcomes across treatment centers and the multiple waves of the pandemic. Lower mortality in children as compared to adults admitted with COVID-19 has been noted worldwide [17]. It may be attributed to the fact that children have a lower ACE2 expression in lungs, lower proinflammatory cytokine response, and are capable of mounting stronger innate immune response [20].
Overall, comorbidities were reported in only 21% of the present pediatric cohort. However, more than half of the kids who died had some coexisting medical condition. This differs slightly from other reports, mainly from tertiary care centers, where a large proportion of the admitted patients had some comorbidities [6]. The clientele of patients and the threshold of admission might be the contributing factors. Coexisting malignancy were associated with higher odds of in-hospital mortality in the present cohort. As in adults, studies evaluating factors associated with mortality in COVID-positive children have found comorbidity to be an important factor [21, 22]. The present results differ from a few studies conducted among pediatric cancer patients, which showed that malignancy was not associated with poor outcomes or mortality among COVID-19 patients [23, 24]. Altered sensorium and WHO ordinal scale ≥ 4 at admission were associated with significantly higher adjusted odds of mortality among the present study subjects. A recently published study from the US also predicted severe disease among pediatric COVID-19 patients who have abnormal vital signs at the time of admission [25].
Furthermore, malnutrition has also been linked with COVID-19 severity [26]. However, lower anthropometric indices were not associated with any adverse outcome in the present cohort. The majority of the admitted children were within normal limits for weight for height or BMI z scores. The present cohort also provides a kaleidoscopic view of the three waves of the COVID-19 pandemic in children in India. Severity or mortality did not differ, but more children in the under-5 group were admitted to the hospital during the third wave. This increase in the proportion of under-5 s coincided with factors such as the unvaccinated status of the young, the reopening of schools, etc.
The current analysis from the National Clinical Registry for COVID-19 is a large, multicentric, widely representative analysis which describes the demographic, anthropometric, clinical and outcome characteristics of hospitalized pediatric COVID-19 patients in India.
Considering this is a record-based study in hospitals, the information collected was reliant on the accuracy of the records maintained. Also, the patients who were transferred to other institutes or who had left against medical advice were not followed up and could not be included in mortality analysis as their outcomes were unknown.
Conclusions
The multicentric cohort of admitted Indian children showed that COVID-19 illness was milder in children than adults, with one-third being asymptomatic. This pattern was consistent over the three waves of the pandemic. Factors associated with higher odds of death were malignancy, altered sensorium, and a WHO ordinal scale ≥ 4 at admission.
References
Girona-Alarcon M, Bobillo-Perez S, Sole-Ribalta A, et al. The different manifestations of COVID-19 in adults and children: a cohort study in an intensive care unit. BMC Infect Dis. 2021;21:87.
UNICEF. Child mortality and COVID-19. UNICEF Data. Available at: https://data.unicef.org/topic/child-survival/covid-19/. Accessed on 25 May 2022.
Arora SK, Shah D, Chaturvedi S, Gupta P. Defining and measuring vulnerability in young people. Indian J Community Med. 2015;40:193–7.
Banerjee M, Pal J, Mondal T, Ghosh T, Nayek K. Clinical profile and short-term outcome of SARS-CoV-2-infected neonates from a Government Medical College in West Bengal, India. J Trop Pediatr. 2022;68:fmac002.
Chaudhuri PK, Chaudhary AK, Prasad KN, Malakar J, Pathak A, Siddalingesha R. An observational study on clinical and epidemiological profile of pediatric patients with coronavirus disease 2019 (COVID-19) presenting with comorbidities at RIMS, Ranchi. J Family Med Prim Care. 2022;11:1493–6.
Jat KR, Sankar J, Das RR, Collaborative Indian Pediatric COVID study group, et al. Clinical profile and risk factors for severe disease in 402 children hospitalized with SARS-CoV-2 from India: Collaborative Indian pediatric COVID study group. J Trop Pediatr. 2021;67:fmab048.
Mussadiq S, Verma RK, Singh DP, Bajpai PK, Begum N, Kumar S. An epidemiological study and trend analysis of laboratory confirmed COVID-19 cases among children in North India. J Family Med Prim Care. 2022;11:542–6.
Ratageri VH, M S, Pawar GR, Illalu S, Wari PK. Clinical profile and outcome of children infected with SARS-CoV-2. Indian J Pediatr. 2021;88:595.
Krishnamurthy S, Kar SS, Dhodapkar R, Parameswaran N. Comparison of COVID-19 infection in children during the first and second wave. Indian J Pediatr. 2022;89:1016–8.
Kumar P, Fadila, Prasad A, et al. Vertical transmission and clinical outcome of the neonates born to SARS-CoV-2-positive mothers: a tertiary care hospital-based observational study. BMJ Paediatr Open. 2021;5:e001193.
More K, Chawla D, Murki S, Tandur B, Deorari AK, Kumar P, National Neonatology Forum (NNF) COVID-19 Registry Group. Outcomes of neonates born to mothers with coronavirus disease 2019 (COVID-19) - National Neonatology Forum (NNF) India COVID-19 Registry. Indian Pediatr. 2021;58:525–31.
Kumar G, Mukherjee A, Sharma RK, National Clinical Registry for COVID-19 Team, et al. Clinical profile of hospitalized COVID-19 patients in first & second wave of the pandemic: Insights from an Indian registry based observational study. Indian J Med Res. 2021;153:619–28.
WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192-7.
Wang JG, Cui HR, Tang HB, Deng XL. Gastrointestinal symptoms and fecal nucleic acid testing of children with 2019 coronavirus disease: a systematic review and meta-analysis. Sci Rep. 2020;10:17846.
Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-33.e3.
van Halem K, Bruyndonckx R, van der Hilst J, et al. Risk factors for mortality in hospitalized patients with COVID-19 at the start of the pandemic in Belgium: a retrospective cohort study. BMC Infect Dis. 2020;20:897.
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–95.
Rao S, Gavali V, Prabhu SS, et al. Outcome of children admitted with SARS-CoV-2 infection: experiences from a pediatric public hospital. Indian Pediatr. 2021;58:358–62.
Sharma AG, Kumar V, Sodani R, et al. Predictors of mortality in children admitted with SARS-CoV-2 infection to a tertiary care hospital in North India. J Paediatr Child Health. 2022;58:432–9.
Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–9.
Ward JL, Harwood R, Smith C, et al. Risk factors for PICU admission and death among children and young people hospitalized with COVID-19 and PIMS-TS in England during the first pandemic year. Nat Med. 2022;28:193–200.
Benedetti C, Waldman M, Zaza G, Riella LV, Cravedi P. COVID-19 and the Kidneys: An Update. Front Med (Lausanne). 2020;7:423.
Węcławek-Tompol J, Zakrzewska Z, Gryniewicz-Kwiatkowska O, et al. COVID-19 in pediatric cancer patients is associated with treatment interruptions but not with short-term mortality: a Polish national study. J Hematol Oncol. 2021;14:163.
de Rojas T, Pérez-Martínez A, Cela E, et al. COVID-19 infection in children and adolescents with cancer in Madrid. Pediatr Blood Cancer. 2020;67:e28397.
Martin B, DeWitt PE, Russell S, et al. Characteristics, outcomes, and severity risk factors associated with SARS-CoV-2 infection among children in the US National COVID cohort collaborative. JAMA Netw Open. 2022;5:e2143151.
Mertens E, Peñalvo JL. The burden of malnutrition and fatal COVID-19: A global burden of disease analysis. Front Nutr. 2021;7:619850.
Acknowledgement
National Clinical Registry for COVID-19 Team: Puspendra Mishra, National Institute of Medical Statistics, Indian Council of Medical Research, Delhi, India; Joseph L. Mathew, Sourabh Dutta, Naveen Sankhyan, Postgraduate Institute of Medical Education & Research, Chandigarh, India; Shachi Ganantra, Nilay N. Suthar,Smt. NHL, Municipal Medical College, Ahmedabad, Gujarat, India; Sanjeev Misra, Kuldeep Singh, All Indian Institute of Medical Sciences, Jodhpur, Rajasthan, India; Rajarao Mesipogu, Mohammed Ayaz Mohiuddin, Vinaya Sekhar Aedula, Gandhi Medical College, Telangana, India; Pankaj Kumar Kannauje, Ajit Kumar, All Indian Institute of Medical Sciences, Raipur Chhattisgarh, India; Gurmeet Kaur, Mary John, Christian Medical College, Ludhiana, Punjab, India; Anuroop Sahu, Naveen Dulhani, Late BRK Memorial Medical College, Jagdalpur, Chhattisgarh, India; Simmi Dube, Jyotsna Shrivastava, Neha Shrivastava, Gandhi Medical College, Bhopal, Madhya Pradesh, India; U. K.Ojha, R. R. Jha, Avinash Kumar, Shaheed Nirmal Mahato Medical College, Dhanbad, Jharkhand, India; Arunansu Talukdar, Mihir Sarkar, Medical College and Hospital, Kolkata, West Bengal, India; Himesh Barman, Star Pala, Annie B. Khyriem, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India; Rakesh Gupta, Rashmi Upadhyay, Government Institute of Medical Sciences, Greater Noida, Uttar Pradesh, India; Mangala Rao, Ratnamala Choudhury, St. Johns Medical College, Bengaluru, Karnataka, India; Lipilekha Patnaik, Jagdish Prasad Sahoo, Institute of Medical Sciences & SUM Hospital, Siksha ‘O’ Anusandhan deemed to be University, Bhubaneswar, Odisha, India; Amit Kumar Satpathy, Sourin Bhuniya, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India; Sachin K. Shivnitwar, Shubhangi Kanitkar, Dr D Y Patil Medical College, Hospital and Research Center, Pune, Maharashtra, India; Mohammed Shameem, Shariq Ahmed, Nazish Fatima, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, Uttar Pradesh, India; Subhasis Mukherjee, Susenjit Mallick, Priyanka Ghosh, College of Medicine and Sagore Dutta Hospital, Kolkata, West Bengal, India; Kundan Mittal, Jagjit Singh Dalal, Pandit Bhagwat Dayal Sharma Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India; Partha Sarathi Bhattacharya, Arpita Bhattacharya, Soumyadip Chatterji, Tata Medical Centre, Kolkata, West Bengal, India; Amit Patel, Surabhi Madan, CIMS Hospital, Ahmedabad, India; Kala Yadav M. L, Chikkanarasa Reddy P. S, Bowring & Lady Curzon Medical College & Research Institute, Bangalore, Karnataka, India; YS Raju, Nizam’s Institute of Medical Sciences, Punjagutta, Hyderabad, Telangana, India; Revanasiddappa Bhosgi, Santosh Algur, Gulbarga Institute of Medical Sciences, Kalburagi, Karnataka, India; Lisa Sarangi, Hi-Tech Medical College and Hospital, Bhubaneswar, Odisha, India; M. Pavan Kumar, A. Bikshapathi Rao, Kakatiya Medical College and MGM Hospital Warangal, Telangana, India; Ashish Pathak, RD Gardi Medical College, Ujjain, Madhya Pradesh, India; Arti Shah, SMT. B.K. Shah Medical Institute & Research Centre & Dhiraj Hospital Vadodara, Ahmedabad, India; Geet Gunjan, GMERS Medical College Himmatnagar, Gujarat, India; Sudhir Bhandari, Abhishek Agrawal, SMS Medical College, Jaipur, Rajasthan, India; Nikita Sharma, Rajaat Vohra, Mahatma Gandhi Medical College, Jaipur, Rajasthan, India; Maninder Singh Dhaliwal, Medanta-The Medicity, Gurugram, Haryana, India; Kalyan Kumar, Sudhabala, ESIC medical College, Sanathnagar, Hyderabad, Telangana, India; Nyanthung Kikon, Department of Health & Family Welfare, Government of Nagaland, Nagaland, India; Shikha Malik; All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India; Soumitra Ghosh, Avijit Hazra, Institute of Postgraduate Medical Education & Research, Kolkata, West Bengal; Himanshu Dandu, King George Medical University, Lucknow, Uttar Pradesh, India; Jigyasa Gupta, Bal Kishan Gupta, Vijay Punia, SP Medical College, Bikaner, Rajasthan, India; Anita Desai, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India.
Funding
The study was funded by the Indian Council of Medical Research, New Delhi.
Author information
Authors and Affiliations
Consortia
Contributions
AT, GK, AM, MJ, SMP, PB, TCB, TDB, LKS, GRM, DS, SP, VVR, BB: Study design, data analysis, data interpretation, and manuscript writing team; AT, GK, AM, GRM, DS, VVR: Monitoring and conduct of the study; MJ, SMP, PB, TCB, TDB & The National Clinical Registry for COVID-19 Team: Patient enrollment, conduct of study, clinical care, and data collection. AM will act as the guarantor for this paper.
Corresponding author
Ethics declarations
Ethical Approval
The approval was obtained from the Central Ethics Committee for Human Research at ICMR as well as from the respective institutional ethics committees of each of the participating centers.
Conflict of Interest
None. AM, AT, GK, LKS, SP, BB are employed by the Indian Council of Medical Research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alka Turuk and Gunjan Kumar are the co-first authors and contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turuk, A., Kumar, G., Mukherjee, A. et al. Evaluation of a Hospitalized Pediatric COVID-19 Cohort from Indian National Clinical Registry of COVID-19. Indian J Pediatr 90, 1000–1007 (2023). https://doi.org/10.1007/s12098-022-04449-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-022-04449-w