Abstract
Objectives
To compare the clinical profile and short-term outcome of children admitted with acute SARS-CoV-2 infection during the first and second waves of the Coronavirus Disease (COVID-19).
Methods
This retrospective study was conducted in a tertiary care setting. A retrospective medical record review of all pediatric patients admitted with confirmed SARS-CoV-2 infection between March 2020 and September 2021 was conducted. Patients’ demographic data, pre-existing comorbidities, mode of presentation, and clinical course in the hospital were noted. The outcome measures were in-hospital mortality, need for intensive care, and invasive mechanical ventilation, duration of ICU, and hospital stay.
Results
One thousand and twenty-four children were recruited, 592 of the first wave and 432 of the second wave. In the second wave, more children were admitted with respiratory distress (OR = 3.38) and neurological manifestations (OR = 4.61). There was a higher requirement of intensive care (OR = 4.2) and invasive mechanical ventilation (OR = 4.17). In-hospital mortality of the second wave was also increased (1.4% vs. 0.1%), but the difference was not statistically significant. Children with neurological comorbidities (OR = 8.73), malnutrition (OR = 3.01), and preterm babies (OR = 6.8) were associated with severe COVID.
Conclusion
The clinical profile of the second wave of COVID-19 in children was different from the first wave, with more respiratory distress and neurological manifestations at presentation. In the second wave, a significant increase in the incidence of severe infections requiring ICU care was observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus Disease (COVID-19) is a novel disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first confirmed case of COVID-19 was reported in Wuhan, Hubei, China, in December 2019 [1]. On 30 January 2020, the World Health Organization declared the coronavirus a Public Health Emergency of International Concern [2]. India is one of the most corona-affected countries in the world. India's first confirmed case of COVID-19 was detected in the southern state of Kerala on 30 January, 2020 [3]. Kerala is one of the most affected states in the country, with overall cases of 50.4 Lakh as of 12 November 2021 [4].
Overall survival and outcomes from critical illness in children with COVID-19 were far better than reported for adults [5]. Pediatric COVID-19 is considered a mild illness, but children with comorbidity often manifest severe disease [5, 6]. There are several studies in adults on the impact of comorbidities on the outcome of acute COVID infection. Similar studies in children are limited.
The second wave of COVID-19 in India was different in presentation than the first wave, with younger demography, lesser comorbidities, and breathlessness in greater frequency [7]. The change in clinical presentation and outcome in the adult population might be due to a better understanding of the disease process, availability of various treatment options, including monoclonal antibody cocktail, newer mutations of circulating viral strains, and widespread vaccination coverage. It is not known whether these factors influence the profile and outcomes in children. There are only limited reports describing clinical characterization and outcome in children during the first and second waves of the COVID-19 in India.
This study aimed to compare the demographic data, clinical characteristics, and short-term outcomes of children admitted with SARS-CoV-2 infection between the first and second waves of the pandemic.
Material and Methods
This retrospective study was conducted in a tertiary care setting. The study was approved by the institutional ethics committee.
The children under 16 y of age admitted with acute SARS-CoV-2 infection with nasopharyngeal swab specimen positive for either one of the following—rapid antigen test, real-time polymerase chain reaction (RT-PCR), TrueNat or GeneXpert, from March 2020 to September 2021 were included.
A retrospective medical record review of all pediatric patients admitted with laboratory-confirmed acute SARS-CoV-2 infection between March 2020 and September 2021 was conducted. The admissions from March 2020 to March 2021 were considered as the first wave and, admissions from April 2021 to September 2021 were considered as the second wave of the pandemic [4]. Patients demographic data, pre-existing comorbidities, mode of presentation, clinical course in the hospital in terms of organ failure, maximum respiratory support and development of shock requiring inotropic support, COVID-19–related multisystem inflammatory syndrome in children (MIS-C), and pharmacotherapies received were noted. The outcome measures were in-hospital mortality, need for intensive care unit (ICU) and invasive mechanical ventilation, duration of ICU, and hospital stay.
Clinical categories (category A, B, and C) were based on the state guidelines for testing, quarantine, hospital admission, and discharge [8]. Acute kidney injury (AKI) was defined by KDIGO staging [9]. A z score of coronary artery diameter with a value of more than 2 was considered to have coronary artery dilatation/aneurysm [10]. Children fulfilling CDC criteria were diagnosed with multisystem inflammatory syndrome in children (MIS-C) [11].
All statistical analyses were performed by SPSS statistics software V26. Descriptive statistics were used to analyze demographic characteristics, clinical profile, and prevalence of pre-existing comorbidities for each of the two waves of the pandemic. Categorical data were presented as counts and percentages; continuous data were presented as mean and standard deviation (SD). Pearson chi-square test and Student t-test were used for comparing the characteristics and outcome of the first and second waves, as appropriate.
Comparisons of COVID-19–related organ involvement and clinical outcomes between the first and second wave were reported as odds ratio (OR) along with 95% confidence interval (CI). A p value less than 0.05 was considered statistically significant. The clinical outcomes of children with comorbidities and without comorbidities were also statistically analyzed.
Results
A total of 1024 children were admitted with SARS-CoV-2 infection from March 2020 to September 2021. The number of children admitted during the first and second waves was 592 and 432, respectively. The first wave peaked in August–September 2020, and the second wave peaked in May 2021. The demographic details of the study population and categorization of cases are displayed in Table 1. In the first wave, 49 (8.3%) children were of category C. In the second wave, 106 (24.5%) were of category C.
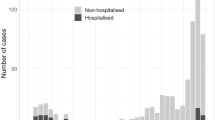
The overall common presenting symptoms were fever (62.6%), diarrhea (11.5%), and cough (10.1%). The mean duration of fever at presentation was 1.5 d. In Fig. 1, the clinical profile of children admitted during the two waves is elucidated. About 94.5% of the children had a history of contact with COVID-19 cases.
The clinical profile of children admitted with acute SARS-CoV-2 infection during the first wave (darker shade) is compared with that of the second wave (lighter shade) of the pandemic. First wave - March 2020 to March 2021; Second wave - April 2021 to September 2021. The incidence of breathlessness, seizures, abdominal pain, vomiting, and shock was increased in the second wave
Overall, respiratory support was needed in 37 children; of which, noninvasive modalities were used in 24 (2.3%), and invasive mechanical ventilation was used in 13 children (1.3%). The requirement of ICU admission had increased from 46 (7.7%) to 115 (26.6%) from the first to the second wave of the pandemic.
Compared to the first wave, the number of children presenting with respiratory distress at admission had increased in the second wave. The requirement for respiratory support had also significantly increased. The incidence of shock had also increased in the second wave. The cardiac involvement like coronary artery dilatation, left ventricular dysfunction, and elevated serum cardiac markers were not significantly raised.
Children who developed seizures, focal deficits, or encephalopathy were considered to have central nervous system (CNS) involvement. Ninety-four children presented with febrile seizures, including 7 with status epilepticus, 10 had encephalopathy. One child had an ischemic stroke causing left hemiparesis, and 1 child developed acute brainstem necrotizing encephalopathy. A significant surge in CNS involvement was recognized in the second wave. Among children with neurological comorbidities, 17 children had CNS involvement. A significant risk of developing COVID related neurological manifestations in children with neurological comorbidities was observed (p value < 0.01; OR 7.52; 95% CI = 3.8 to 14.6).
The comparison of COVID-19–related organ involvement and clinical outcome of children between the first and second waves is presented in Table 2. The number of severe COVID infections was significantly increased in the second wave. Children with comorbidities, requirement of ICU admission, and invasive mechanical ventilation were also increased in the second wave.
The treatment summary of children admitted during the first and second waves of COVID-19 has been narrated in Table 3. Intravenous immunoglobulin (IVIG) was administered to 15 children. The indications of IVIG include MIS-C in 10 children, severe acute respiratory distress syndrome (ARDS) in 2 children, severe viral myocarditis in 1 child, acute brainstem necrotizing encephalopathy in 1 child, and immunodeficiency in 1 child. Remdesivir was given to 10 children; of which, 9 children survived, indications being pneumonia with severe ARDS in 9 children and acute encephalitis in 1 child. Out of those 9 children, 6 children were on noninvasive ventilation (NIV) support and, 3 children were on invasive mechanical ventilation. Remdesivir was started within 5 d of illness in all children. The number of children who received methylprednisolone pulse was 20; of which, 18 children survived. The indications include MIS-C in 19 children and acute brainstem necrotizing encephalopathy in 1 child.
One thousand and eight (98.4%) children were discharged without any sequelae. Nine children were discharged with sequelae; of which, 7 were coronary artery aneurysms and 2 were neurological—hemiparesis secondary to multifocal infarct and quadriparesis secondary to acute necrotizing encephalopathy.
The overall in-hospital mortality was 7, with 1 in the first wave and 6 in the second wave. Of 7 children, 6 had pre-existing comorbidities; 3 had life-limiting illnesses (chronic kidney disease with chronic liver disease, medulloblastoma, and storage disorder); 2 were preterm babies, 1 with severe acute malnutrition. One mortality in the noncomorbidity group was a 4-mo-old infant who presented with myocarditis with severe left ventricular dysfunction, succumbed to death within 48 h of admission. The primary cause of death was COVID pneumonia with severe ARDS in 2 children, severe viral myocarditis in 1 child, and multiorgan failure in 4. The in-hospital mortality rate was 0.1% in the first wave and 1.4% in the second wave, but the rise was statistically not significant (p value = 0.05). Four out of 7 expired children were infants, but no statistical significance was noted between infancy and mortality (p value = 0.12).
Eighteen percent (n = 185) of the study population had pre-existing comorbidities. The most common being malignancy (n = 59) followed by malnutrition (n = 48) and neurological comorbidities (n = 40). The clinical outcomes of children with comorbidities and without comorbidities were statistically analyzed. Among the children admitted with comorbidities, 24% had developed severe COVID, whereas 13% of children without comorbidities had developed severe COVID. The in-hospital mortality in children with comorbidities was 3.2%, while only 0.1% in children without comorbidities.
The significant increase in shock (p value < 0.01; OR 3.51; 95% CI = 1.8 to 6.83), requirement of respiratory support (p value < 0.01; OR 8.36; 95% CI = 4.21 to 16.6), and AKI (p value = 0.01; OR 6.15; 95% CI = 1.36 to 27.75) were noted in the children with comorbidities. Severe COVID (p value < 0.01; OR 2.13; 95% CI = 1.44 to 3.15), need for ICU care (p value < 0.01; OR 2.49; 95% CI = 1.7 to 3.64), invasive mechanical ventilation (p value < 0.01; OR 14.25; 95% CI = 3.81 to 53.16), and in-hospital mortality (p value < 0.01; OR 28.36; 95% CI = 3.36 to 234.75) were also significantly increased.
The association of various comorbidities with severe COVID in all children (n = 1024) admitted with SARS-CoV-2 infection is presented in Table 4. Neurological comorbidities, malnutrition, and preterm babies were associated with severe COVID. The variables with a p value of < 0.05 were included in the logistic regression to assess the effect of comorbidities on the likelihood of occurrence of severe COVID. The Independent predictors of severe COVID after logistic regression analysis were neurological comorbidity (p value < 0.01; OR 14.6; 95% CI = 5.5 to 38.47) and preterm babies (p value < 0.01; OR 5.9; 95% CI = 1.81 to 19.29).
Discussion
In India, the first wave of COVID-19 started rising considerably towards the end of June 2020 and peaked in about the middle of September 2020 followed by a low infection rate period during January–February 2021, and a distinct resurgence peaked in the first week of May 2021. The second wave had a steeper rise and higher peak with more cases than the first wave in India and Kerala. A similar trend was witnessed in the present study population also.
Even though the number of admissions was more during the first wave, nearly 92% of admissions were nonsevere cases. During the initial period of the first wave, mild cases were also hospitalized for observation. So the proportion of mild cases was more in the first wave. In the subsequent months, mild cases were kept in home isolation and were telephonically monitored for red flag signs [8]. The frequency of severe cases has increased from the first to the second wave.
In a multinational study, Götzinger et al. noted a median age of 5 y and a sex ratio of 1·15 males per female [12]. The median age noted in the present study was 3 y, as observed in other studies [6]. There was no gender predisposition recognized in the first wave, but a slight male predominance was recorded in the second wave with a male:female ratio of 1.2:1.
About 94% of children in the present study population were household contacts of adults with SARS-CoV-2 infection, which might be signifying that children were not the primary source of infection. Studies have shown that children acquire SARS-CoV-2 infection from an adult contact, with minimal secondary transmission from children [13].
Cytokine storm, endothelitis, coagulopathy, and neurotropism of spike protein in SARS-CoV-2 are attributed to the neurological manifestations associated with COVID-19 infection [14, 15]. There was a significant surge in CNS involvement in the second wave, with seizure being the most common manifestation. LaRovere et al. have observed more neurologic manifestations in children with underlying neurologic disorders as observed in the present study [16]. Further studies are needed to confirm the surge in COVID-19–related neurological manifestations by comparing with the background incidence of non-COVID neurological illnesses.
The comparison of outcome measures revealed more morbidity and mortality in children with comorbidities as observed in other studies [17, 18]. Despite being the most common comorbidity in the present study population, there was no statistically significant association between malignancy and severe COVID. Millen et al. commented that pediatric cancer patients with SARS-CoV-2 infection do not have an increased risk of severe illness compared to the general population [19]. Outcomes of pediatric cancer patients from lower middle-income countries have also been found favorable [20].
Neurological comorbidities were associated with severe disease, as observed in other studies also [21]. Malnutrition [22, 23] was significantly associated with severe illness in the present study. Kulkarni et al. [23] have recognized malnutrition and anemia to have an association with severe disease. Childhood obesity is also considered a risk factor of severe COVID-19 [18, 21], but it was not observed in the present study.
Antoon et al. observed that older children and adolescents developed severe forms of illness when hospitalized [21]. In the present study, older children had no increased risk of developing severe COVID. Increased mortality was observed in infancy in the present study, but it was not statistically significant.
The second wave was characterized by a significant increase in the requirement of respiratory support and intensive care. This might be due to the more virulent and transmissible circulating variants in the second wave, B.1.1.7 and B.1.617 [24], and children are yet to get their vaccination. Like in adults, the mortality increased in the second wave, but was not statistically significant [7].
The United States Food and Drug Administration has approved the use of the COVID-19 vaccine in children aged 5 y and above on 29 October 2021. The trials have shown that the vaccine is about 91% effective in preventing COVID-19 infection [25]. In view of the increased severity of COVID-19 in the second wave as observed in the present study, it is the need of the moment to consider vaccination in children, especially in those with comorbidities.
The limitations of the present study are that it is a retrospective study, and only the association can be determined; it is a hospital-based study; more children with severe illness and comorbidities were managed in the present center. Further multicentric and population-based studies are required to make the results more generalized. This study has reported the short-term in-hospital outcomes only.
Conclusion
The clinical profile of the second wave of COVID-19 in children was different from the first wave, with more respiratory distress and neurological manifestations at presentation. A significant increase in the incidence of severe infections requiring ICU care with respiratory and inotropic support was also recognized. Children with neurological comorbidities, malnutrition, and preterm babies exhibit more adverse outcomes.
References
Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: An overview. J Chin Med Assoc. 2020;83:217–20.
MacKinnon M, VanderKlippe N, Robertson G. Flattery and foot dragging: China’s influence over the WHO under scrutiny. The Globe and Mail. 2020. Available at: https://www.theglobeandmail.com/world/article-flattery-and-foot-dragging-chinas-influence-over-the-who-under/. Accessed on 17 Nov 2021.
Andrews MA, Areekal B, Rajesh KR, et al. First confirmed case of COVID-19 infection in India: a case report. Indian J Med Res. 2020;151:490–2.
Department of Health and Family Welfare, Government of Kerala. Kerala Health - Covid 19 Dashboard. eHealth Kerala. Available at: https://covid19.kerala.gov.in/dboard.php. Accessed on 17 Nov 2021.
Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–73.
Rao S, Gavali V, Prabhu SS, et al. Outcome of children admitted with SARS-CoV-2 infection: experiences from a pediatric public hospital. Indian Pediatr. 2021;58:358–62.
Kumar G, Mukherjee A, Sharma RK, et al. National Clinical Registry for COVID-19 Team. Clinical profile of hospitalized COVID-19 patients in first & second wave of the pandemic: Insights from an Indian registry based observational study. Indian J Med Res. 2021;153:619–28.
Department of Health and Family Welfare, Government of Kerala. Revised guidelines for testing, quarantine, hospital admission and discharge for COVID-19 based on current risk assessment. 2020. reg_12032020. Available at: https://dhs.kerala.gov.in/wp-content/uploads/2020/03/reg_12032020.pdf. Accessed on 17 Nov 2021.
Ad-hoc working group of E, Fliser D, Laville M, et al. A European renal best practice (ERBP) position statement on the kidney disease improving global outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27:4263–72.
McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–99.
CDC. Multisystem Inflammatory Syndrome (MIS). Centers for Disease Control and Prevention. 2020. Available at: https://www.cdc.gov/mis/mis-c/hcp/index.html. Accessed on 10 May 2022.
Götzinger F, Santiago-García B, Noguera-Julián A, et al. ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–61.
Williams PCM, Howard-Jones AR, Hsu P, et al. SARS-CoV-2 in children: spectrum of disease, transmission and immunopathological underpinnings. Pathology. 2020;52:801–8.
Veleri S. Neurotropism of SARS-CoV-2 and neurological diseases of the central nervous system in COVID-19 patients. Exp Brain Res. 2022;240:9–25.
Maury A, Lyoubi A, Peiffer-Smadja N, de Broucker T, Meppiel E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: a narrative review for clinicians. Rev Neurol (Paris). 2021;177:51–64.
LaRovere KL, Riggs BJ, Poussaint TY, et al. Overcoming COVID-19 Investigators. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–47.
Harman K, Verma A, Cook J, et al. Ethnicity and COVID-19 in children with comorbidities. Lancet Child Adolesc Health. 2020;4:e24–5.
Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246–56.
Millen GC, Arnold R, Cazier JB, et al. Severity of COVID-19 in children with cancer: report from the United Kingdom paediatric coronavirus cancer monitoring project. Br J Cancer. 2021;124:754–9.
Ramaswamy A, Nayak L, Roy Moulik N, et al. COVID-19 in cancer patients on active systemic therapy - Outcomes from LMIC scenario with an emphasis on need for active treatment. Cancer Med. 2020;9:8747–53.
Antoon JW, Grijalva CG, Thurm C, et al. Factors associated with COVID-19 disease severity in US children and adolescents. J Hosp Med. 2021;16:603–10.
Saha J, Chouhan P. Do malnutrition, pre-existing morbidities, and poor household environmental conditions aggravate susceptibility to Coronavirus disease (COVID-19)? A study on under-five children in India. Child Youth Serv Rev. 2021;128:105962.
Kulkarni R, Rajput U, Dawre R, et al. Severe malnutrition and anemia are associated with severe COVID in infants. J Trop Pediatr. 2021;67:fmaa084.
COVID-19 B.1.617 Variant of Concern – What We Know So Far. Available at: https://www.publichealthontario.ca/-/media/documents/ncov/covid-wwksf/2021/06/wwksf-covid-19-b1617.pdf?sc_lang=en. Accessed on 10 May 2022.
COVID-19 Vaccines. FDA. 2021. Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines. Accessed on 17 Nov 2021.
Acknowledgements
The authors would like to gratefully acknowledge all unit chiefs Dr Sankar VH, Dr Devakumar VK and Dr Leela Kumari, Department of Pediatrics, SATH, Thiruvananthapuram, Dr Lakshmi S, HOD, Pediatric Cardiology, Dr Mary Iype, HOD, Pediatric Neurology, Dr Susan Uthup, HOD, Pediatric Nephrology, Government Medical College, Thiruvananthapuram, who were involved in patient care; Microbiology Department, Government Medical College, Thiruvananthapuram; Dr Jedidiah Samraj M for his support and guidance in statistical analysis and original draft preparation.
Funding
None.
Author information
Authors and Affiliations
Contributions
SM designed the study and participated in the acquisition, analysis, and interpretation of data, drafting of the manuscript; Bindu S conceptualized the study and participated in the statistical analysis, interpretation of data, and critical revision of the manuscript; SS conceptualized the study, analyzed the data, and participated in manuscript writing; GK participated in the acquisition, analysis, and interpretation of data; Bindusha S participated in the statistical analysis and critical revision of the manuscript; AKAS supervised the study and contributed to the critical revision of the manuscript for intellectual content. All authors approve the final version of the manuscript and are accountable for all aspects related to the study. AKAS will act as the guarantor for this paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muthusamy, S., Sarojam, B., Sugunan, S. et al. Clinical Profile and Short-Term Outcome of Children with Acute SARS-CoV-2 Infection During the First and Second Waves of the Pandemic. Indian J Pediatr 90, 443–449 (2023). https://doi.org/10.1007/s12098-022-04193-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-022-04193-1