Abstract
Objective
To characterize thoracic (lung and diaphragm) ultrasound findings in children < 2 y with bronchiolitis, evaluate correlation between lung ultrasound severity score (USS) and bronchiolitis severity score (BSS), and study the interobserver agreement of USS between study pediatrician and radiologist.
Methods
In this prospective observational study, thoracic ultrasound was performed on children with bronchiolitis by the study pediatrician and USS score was assigned. A radiologist blinded to all clinical information, performed an independent thoracic ultrasound. Demographics, clinical course, and other relevant details were recorded.
Results
Fifty-three children were enrolled; 29/53 patients (54.7%) were classified as mild bronchiolitis and 24/53 (45.2%) had moderate bronchiolitis as per clinical score; 13.2% (7/53) patients had both anterior and posterior subpleural consolidation and went on to require higher respiratory support either in the form of continuous positive airway pressure in 71.4% (5/7), oxygen for > 24 h in 14.2% (1/7), or heated humidified high-flow nasal cannula in 14.2% (1/7). These results were statistically significant (p < 0.001). A statistically significant correlation was found between the USS and type and duration of respiratory support (p value 0.002) and with the mean duration of hospital stay (p value < 0.001). There was significant correlation between the BSS and USS (p < 0.001). There was a very good agreement between the ultrasound findings of study pediatrician and radiologist (kappa 0.83).
Conclusion
The findings of lung ultrasound (LUS) are not specific for bronchiolitis. However, LUS can be used as a good prognostic tool in patients with bronchiolitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
American Academy of Pediatrics (AAP) defines bronchiolitis as a constellation of clinical symptoms and signs with a viral upper respiratory prodrome followed by increased respiratory effort and wheezing in children less than 2 y of age [1]. Several clinical scoring tools based on these observations have been in widespread use. Even though useful, clinical scores do not accurately indicate the need for higher respiratory support nor have any predictive ability with regard to course of the disease and length of hospitalization.
In this context, lung ultrasound (LUS) has emerged as a valuable bedside modality that can enable dynamic visualization of lung pathology. It may also facilitate prompt identification of alternative diagnoses warranting differential therapeutic approach. Several researchers have demonstrated its utility in pneumonia, acute respiratory distress syndrome (ARDS), pleural effusion, pneumothorax, etc. [2,3,4,5,6,7,8,9]. However, applicability in children with bronchiolitis is still a contemporary concept. So far, very few studies have explored the utility of LUS in hospitalized children with bronchiolitis [10, 11]. Emerging reports indicate that LUS findings have a significant correlation with the disease severity and can also predict duration of respiratory support including oxygen therapy in some children [12, 13].
However, only one study to date has assessed the utility of diaphragmatic ultrasound in predicting the clinical course of bronchiolitis [14]. The aim of the present study was to elucidate the thoracic ultrasound (including lung and diaphragm) findings in children < 2 y of age with bronchiolitis and correlate these findings with indices of morbidity in terms of need for higher respiratory support and length of hospitalization. Secondary objectives were (1) to compare the agreement between lung ultrasound score (USS) and clinical scoring: bronchiolitis severity score (BSS) (2) to evaluate interobserver reliability between the study pediatrician and trained radiologist.
Material and Methods
This was a prospective observational study conducted from August 2018 to February 2020 in the Department of Pediatrics in a tertiary care hospital in Western Rajasthan, India. Children aged 1–24 mo with a clinical diagnosis of bronchiolitis (AAP definition) consecutively admitted to pediatric emergency were enrolled after parental consent. Exclusion criteria included neonates, patients with hemodynamically significant congenital heart disease, chronic lung disease, and neuromuscular weakness.
The study pediatrician performed the LUS in all enrolled patients within 24 h of admission. Radiologist blinded to all clinical information performed an independent assessment within 2 h of the first sonogram. Positive LUS was defined as the presence of ≥ 1 of the following findings: ≥ 3 B lines per intercostal space, consolidation, and/or pleural abnormalities.
Before commencement of the study, the study pediatrician underwent training in lung and diaphragmatic ultrasound in the radiology department at the authors' institute for consecutive 4 wk. Demographic data and baseline clinical characteristics of all patients which included vital signs, physical examination findings, and bronchiolitis severity score (BSS) were recorded by the clinician in charge of the patient care as per the unit protocol. A dedicated Siemens Acuson X300 PE machine with a high-resolution linear probe (4–12 MHz) and curvilinear probe was arranged during the study period to ensure prompt availability. An independent ultrasonographic assessment of each patient was done within 24 h of admission using a six-zone longitudinal scanning protocol, from apex to the base of the lungs [2, 15]. Images and video recordings were taken in each of the six zones for further review. Additionally, an independent thoracic ultrasound was performed with the same standardized protocol by radiologist. The results were analyzed after study completion to assess for interobserver coefficient.
Neither the study pediatrician nor the radiologist were privy to the clinical scores or treatment administered prior to ultrasonographic assessment. Ultrasonographic assessments were broadly classified as lung ultrasound and diaphragmatic ultrasound findings. A composite ultrasound severity score given by Basile et al. was calculated using the above parameters which, in turn, was used to grade the severity of bronchiolitis as mild (score 1–3), moderate (score 4–6), or severe (score 7–8) [10]. USS was based on the presence or absence of B lines, focal or confluent B lines, presence and extent of interstitial syndrome, and presence of subpleural consolidation anteriorly and/or posteriorly.
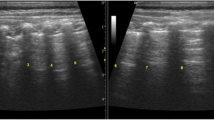
For the procedure, informed parental consent was taken and no sedatives were administered to any patient in the study. Lung ultrasound was performed by dividing each hemithorax into 3 imaginary areas: the anterior—limited by parasternal and anterior axillary lines; the lateral—limited by anterior and posterior axillary line; and the posterior—limited by the posterior axillary line and spinous processes of vertebra. Several longitudinal and transverse sections were taken in these areas and images were saved for future analysis (Fig. 1a, b).
Lung ultrasound (LUS) findings in bronchiolitis. (a) Longitudinal section of lung ultrasound showing pleural line and A lines interrupted by posterior acoustic shadowing of multiple ribs. (b) Transverse section of lung ultrasound with arrow head showing multiple A lines, the reverberation artifacts which are equidistant from the pleural line. (c) Ultrasound image showing multiple focal B lines in a posterior lung field. (d) Lung ultrasound image showing confluent B lines (arrow). (e) LUS in the posterior lung fields showing subpleural consolidation with multiple echogenic air bronchograms
For the scan of the anterior chest wall, the children were made to lie supine and transverse sections were taken from 2nd to 11th intercostal spaces and longitudinal sections in the parasternal, midclavicular, anterior, and midaxillary lines.
For the scan of the posterior chest wall, they were made to lie in the prone position; and transverse sections were taken below the scapula and longitudinal along the paravertebral, scapular and posterior axillary lines.
The following points were assessed on lung ultrasound:
-
The presence of normal lung sliding with or without A lines—normal pattern (Fig. 1b)
-
Presence of focal multiple B lines (black and white lung) (Fig. 1c)
-
Presence of confluent B lines (white lung) (Fig. 1d)
-
Presence of hypoechoic/hyperechoic area with ill-defined margins at the subpleural region—subpleural consolidation (< 1 cm, > 1 cm) (Fig. 1e)
-
Irregularities in the pleural line—thickening greater than 0.5 mm, evidence of small subpleural consolidations obscuring the pleural line or irregular or coarse appearance of the pleural line.
Ultrasound severity score was calculated using the above parameters which, in turn, was used to grade the severity of bronchiolitis as mild (score 1–3), moderate (score 4–6), or severe (score 7–8). Patients were followed up till discharge and their treatment details and outcomes were recorded.
Diaphragmatic ultrasound was performed by placing the transducer in the intercostal space above the 10th rib in the midaxillary or anterior-axillary line and directing the ultrasound beam perpendicular to the diaphragm. The diaphragm was identified as a muscular hypoechoic band between two clear bright parallel lines of the pleural and peritoneal membranes (Fig. 2a).
Diaphragm thickness measurement: D1- thickness at the end of inspiration; D2—thickness at the end of expiration (a) and diaphragmatic movements: A-B indicates displacement of diaphragm, i.e., diaphragmatic excursion (DE); A-C indicates inspiratory slope; A-D represents inspiratory time; and A-E represents duration of respiratory cycle (b) in M-mode ultrasound
The diaphragm thickness was measured in time-motion mode, and the following measurements were noted (Fig. 2a, b).
-
TEE: Thickness at the end of expiration (in mm).
-
TEI: Thickness at the end of inspiration (in mm).
-
Diaphragmatic excursion (DE): DE was obtained by measuring the vertical distance between the upper border of the liver at the end of expiration and the upper border of the liver at the end of inspiration (in mm).
-
IS: Inspiratory slope (the speed of diaphragmatic contraction measured in cm/s)
-
TF: Thickening fraction (TF = TEI − TEE/TEE)
Additional parameters documented were the need for higher respiratory support, pediatric intensive care unit (PICU) admission, length of hospitalization and duration of oxygen therapy.
Data was entered in a Microsoft Excel datasheet and analyzed using SPSS version 23. Categorical data was represented in the form of frequencies and proportions. Chi-square test was used as a test of significance for qualitative data. Continuous data were represented as mean and standard deviation. Student t test was used as a test of significance to identify the mean difference between two quantitative variables. Pearson correlation was done to find the correlation between two quantitative variables, and was interpreted as a positive correlation if the coefficient was between 0 and 1, and negative correlation if it was between 0 and −1.
Results
During the study period, a total of 56 children who met the AAP criteria for bronchiolitis were encountered. Of these 56, 2 were excluded from the study as per the preset criteria laid down in the protocol and 1 refused to give consent. Thus, a total of 53 children were enrolled; of which, 37 (69.8%) were male. Figure 3 shows a flow diagram depicting the flow of participants in the study; 29/53 patients (54.7%) were classified as mild bronchiolitis and 24/53 (45.2%) had moderate bronchiolitis as per clinical score. Of the 53 patients, mean age in mild cases was 7.69 mo (± 3.47), and 7.15 mo (± 3.97) in moderate cases. Mean duration of symptoms prior to hospital visit was 4.02 ± 2.63 d. The baseline clinical characteristics are detailed in Table 1. There was no mortality in the present study cohort.
In the present study, 88.6% (47/53) patients had abnormal lung ultrasound. These included confluent B lines in 45.2% (24/53), multiple focal B lines in 7.5% (4/53), posterior subpleural consolidation of < 1 cm size in 11.3% (6/53), > 1 cm in size in 30.1% (16/53), anterior subpleural consolidation of < 1 cm in 5.6% (3/53), > 1 cm in 13.2% (7/53).
Additionally, the sonographic measures of diaphragmatic function in children with bronchiolitis were evaluated (Fig. 2). Patients with moderate bronchiolitis, clinically, had slightly lower diaphragmatic excursion (DE) compared to those with mild bronchiolitis (13.01 ± 3.42 vs. 14 ± 3.87, p 0.597). Similar observations were made with regard to thickening fractions which were lower amongst moderate bronchiolitis when compared to mild bronchiolitis (0.18 ± 0.11 vs. 0.23 ± 0.11, p 0.11). However, the results were not statistically significant. There was no significant correlation found between inspiratory slope, thickness at the end of inspiration with ultrasound severity score, or bronchiolitis severity score. Also, there was no significant association between the thickening fraction and inspiratory slope with respiratory support or duration of hospital stay.
In patients with moderate bronchiolitis as per BSS, 8/24 (33.3%) required CPAP, 1/24 (4.15%) required HHHFNC, 3/24 (12.55) required oxygen by nasal prongs for > 24 h, and 11/24 (45.8%) required oxygen by nasal prongs for < 24 h. A statistically significant association was found between BSS and type of respiratory support (p 0.001) (Table 2).
Ultrasound severity score and the type of respiratory support needed were also correlated, as depicted in Table 2. In this study, 18/53 (33.9%) patients required only supportive care, which included saline nasal drops, hydration and observation, 23/53 (43.3%) patients required oxygen by nasal prongs for < 24 h, 3/53 (5.6%) patients required oxygen by nasal prongs for > 24 h, 9/53 (16.9%) patients required noninvasive ventilation including continuous positive airway pressure (CPAP) in 8/53 (15.0%) and heated humidified high flow nasal cannula (HHHFNC) in 1/53 (1.8%) patients. A statistically significant association was found between USS and type of respiratory support (p 0.002).
In the present study, 13.2% (7/53) patients had both anterior and posterior subpleural consolidation and went on to require higher respiratory support either in the form of CPAP in 71.4% (5/7), oxygen by nasal prongs for > 24 h in 14.2% (1/7), or HHHFNC in 14.2% (1/7) patients. Results were statistically significant (p < 0.001).
Those who had mild bronchiolitis (28/53) on ultrasound severity score had mean hospital stay of 1.32 ± 1.09 d, whereas those with moderate bronchiolitis (19/53) had 2.89 ± 0.99 d. Those with normal lung ultrasound required admission for a mean duration of 1.33 ± 1.2 d. Results were statistically significant (p < 0.001).
According to the present study, 29/53 had mild bronchiolitis and 24/53 had moderate bronchiolitis according to BSS. However, ultrasonographic scores were classified as normal in 6/53, mild bronchiolitis in 28/53, and moderate bronchiolitis in 19/53. There was significant correlation between the BSS and USS (p < 0.001).
Moreover, very good agreement (0.83) according to kappa statistics was found between the USS done by the study pediatrician and radiologist.
Discussion
Point-of-care ultrasound (POCUS) is now an essential part of evidence-based management of many respiratory conditions [2,3,4,5]. A recent meta-analysis stated that POCUS has superior diagnostic accuracy in pediatric pneumonia compared to conventional chest radiographs [16]. Caiulo et al. in their study showed that LUS was superior in identification of lung abnormalities compared to chest radiographs and these findings correlated well with the clinical course of the disease. Thus, unnecessary radiation exposures, overzealous use of antibiotics, and related costs can be prevented with the use of LUS in bronchiolitis [12].
The present study demonstrated that LUS is able to identify several lung pathologies in bronchiolitis. Prevalence of positive LUS in the present cohort was 88.6%, which is slightly lower than previously published reports [10, 12]. This may be due to the smaller cohort of mild–moderate bronchiolitis, and no case of severe bronchiolitis in the present study.
Predominance of posterior regional abnormalities was observed on LUS. Basile et al. documented posterior chest abnormalities in 86% of their cohort [10]. Likewise was observed in the study by Garrotea et al. [11]. This may be due to gravitational effects and formation of atelectatic zones in posterior regions of lungs in infants due to obligate supine positioning.
Also, it was found that children with both anterior and posterior consolidations had greater need for supplemental oxygen and noninvasive ventilation. Some studies have found an etiological link between this particular finding and respiratory syncytial virus pathogen [13]. Since virological surveillance were not performed in this study, this remains an unexplored dimension. By contrast, another study with similar observations could not ascertain the association between specific viruses and lung pathology pattern [17].
Several studies have concurred that pathological findings in LUS tend to correlate with disease severity and resolution with clinical recovery. However, very few studies have explored the potential role of diaphragmatic function in children with bronchiolitis. Notably, Buonsenso et al. [14], found that a lower TF implied disease severity and greater requirement of respiratory support. They concluded that TEI was strongly associated with the length of oxygen requirement. This aligns well with the results of the present study, as there was a trend towards lower DE (13.01 ± 3.42 vs. 14 ± 3.87, p 0.597) and lower TF (0.18 ± 0.11 vs. 0.23 ± 0.11, p 0.11) in those with moderate disease.
As bronchiolitis is a clinical diagnosis, none of the imaging techniques are recommended for diagnosing bronchiolitis (including chest radiograph). In the present study chest radiography was not done in all the children, hence could not be compared with LUS findings.
The present study has some significant strengths. As far as the authors know, this is the only study from India to evaluate the function of diaphragm in children with bronchiolitis. Additionally, real-time analyses could be performed by operators of varying skill in sonography with excellent interobserver agreement. This further supports the idea that LUS is a valuable resource that can be explored in different settings by clinicians with relatively short learning curve.
Nevertheless, some limitations of the present study should be addressed. Firstly, the major limitation in the present study is that no patient with severe bronchiolitis could be met during the study period. Secondly, a convenience sampling was taken thus limiting the sample size, hence, reducing the overall generalizability of results. Another major limitation is the lack of sensitivity and specificity of lung ultrasound findings in the diagnosis of bronchiolitis. However, lung ultrasound can be used as a prognostic marker, as it correlates well with the duration of hospital stay and respiratory support needed.
Conclusion
The findings of lung ultrasound are not specific for bronchiolitis; however, a good correlation was found between these findings and duration of hospital stay, and type of respiratory support needed. Hence, LUS can be used as a good prognostic, rather than a diagnostic tool, in bronchiolitis. However, larger studies are needed to develop systematic, reproducible, and validated screening protocols to ensure that its full utility is achieved.
References
Subcommittee on diagnosis and management of bronchiolitis. diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–93.
Cattarossi L. Lung ultrasound: its role in neonatology and pediatrics. Early Hum Dev. 2013;89:S17–9.
Smargiassi A, Inchingolo R, Soldati G, et al. The role of chest ultrasonography in the management of respiratory diseases: document II. Multidiscip Respir Med. 2013;8:55.
Raimondi F, Cattarossi L, Copetti R. International perspectives: point-of-care chest ultrasound in the neonatal intensive care unit: an italian perspective. Neo Reviews. 2014;15:e2-6.
Mong A, Epelman M, Darge K. Ultrasound of the pediatric chest. Pediatr Radiol. 2012;42:1287–97.
Volpicelli G, Melniker LA, Cardinale L, Lamorte A, Frascisco MF. Lung ultrasound in diagnosing and monitoring pulmonary interstitial fluid. Radiol Med (Torino). 2013;118:196–205.
Yang P-C, Luh K-T, Chang D-B, Yu C-J, Kuo S-H, Wu H-D. Ultrasonographic evaluation of pulmonary consolidation. Am Rev Respir Dis. 1992;146:757–62.
Soldati G, Giunta V, Sher S, Melosi F, Dini C. “Synthetic” comets: a new look at lung sonography. Ultrasound Med Biol. 2011;37:1762–70.
Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact: an ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640–6.
Basile V, Di Mauro A, Scalini E, et al. Lung ultrasound: a useful tool in diagnosis and management of bronchiolitis. BMC Pediatr. 2015;15:63.
Zoido Garrote E, García Aparicio C, Camila Torrez Villarroel C, Pedro Vega García A, Muñiz Fontán M, Oulego Erroz I. Usefulness of early lung ultrasound in acute mild-moderate acute bronchiolitis. A pilot study. An Pediatr (Engl Ed). 2019;90:10–8.
Caiulo VA, Gargani L, Caiulo S, et al. Lung ultrasound in bronchiolitis: comparison with chest X-ray. Eur J Pediatr. 2011;170:1427–33.
Supino MC, Buonsenso D, Scateni S, et al. Point-of-care lung ultrasound in infants with bronchiolitis in the pediatric emergency department: a prospective study. Eur J Pediatr. 2019;178:623–32.
Buonsenso D, Supino MC, Giglioni E, et al. Point of care diaphragm ultrasound in infants with bronchiolitis: A prospective study. Pediatr Pulmonol. 2018;53:778–86.
Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16.
Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714–22.
Buonsenso D, Musolino A, Ferro V, et al. Role of lung ultrasound for the etiological diagnosis of community- acquired pneumonia in children: a prospective study. medRxiv. 2020. https://doi.org/10.1101/2020.10.31.20223867.
Author information
Authors and Affiliations
Contributions
D Krishna, D Khera, NT, BS, BC, VMG, and KS made substantial contributions to the conception and drafting of the work and revising it critically for important intellectual content and finally approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. KS will act as the guarantor for this paper.
Corresponding author
Ethics declarations
Ethics Approval
AIIMS/IEC/2018/668
Consent to Participate
Written informed consent was obtained from the parents/legal guardians.
Consent for Publication
Written informed consent was obtained from the parents/legal guardians.
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krishna, D., Khera, D., Toteja, N. et al. Point-of-Care Thoracic Ultrasound in Children with Bronchiolitis. Indian J Pediatr 89, 1079–1085 (2022). https://doi.org/10.1007/s12098-022-04117-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-022-04117-z