Abstract
Objective
To evaluate the epidemiology of rotavirus gastroenteritis in Haryana post-introduction of rotavirus vaccine. Expanded National rotavirus surveillance network in India reported high burden of rotavirus diarrhea in India. The Government of India introduced the monovalent rotavirus vaccine made in India by Bharat Biotech in the national immunization programme from 2016 onward along with oral polio vaccine (OPV) and Pentavalent vaccines.
Methods
A multi-centric, hospital-based surveillance study in the initial vaccine introducing states was started in a phased manner over a period of 3 y. PGIMS, Rohtak is a tertiary care center and was a part of the surveillance from 2016 to 2019. Children aged 0–59 mo admitted with acute gastroenteritis were enrolled into the surveillance and their stool samples were collected. Samples were tested at Christian Medical College (CMC), Vellore to detect rotavirus and reverse transcription-polymerase chain reaction (RT-PCR) was used for G and P typing.
Results
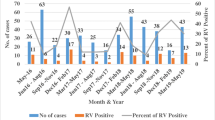
A total of 904 children were enrolled in the present surveillance over a period of 3 y starting 1st July 2016 to 30th June 2019. Stool samples were collected and analyzed for 827 children and out of them 141 samples were positive for rotavirus (17.1%). Maximum rotavirus positivity was observed during the winter months. Rotavirus positivity percentage was observed maximum in 12–23 mo age group. A declining trend was observed in rotavirus positivity from 22.8% in 2016 to 14.5% in 2019. Most common strains of rotavirus isolated were G3P[8] followed by G1P[8].
Conclusion
This study highlights that epidemiology of acute gastroenteritis among children less than 5 y of age in Haryana postintroduction of rotavirus vaccination in the state and the decline in rotavirus positivity from 22.8% in 2016 to 14.5% in 2019.



Similar content being viewed by others
References
Troeger C, Blacker B, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. 2018;18:1191–210 Available at: http://www.sciencedirect.com/science/article/pii/S1473309918303104. Accessed 5 Feb 2019.
Clark A, Black R, Tate J, et al. Estimating global, regional and national rotavirus deaths in children aged <5 years: current approaches, new analyses and proposed improvements. PLoS One. 2017;12:e0183392. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5593200/. Accessed 15 Sep 2020.
Lanata CF, Fischer-Walker CL, Olascoaga AC, et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8:e72788 Available at: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0072788. Accessed 28 Aug 2019.
Liu L, Chu Y, Oza S, et al. National, regional, and state-level all-cause and cause-specific under-5 mortality in India in 2000–15: a systematic analysis with implications for the sustainable development goals. Lancet Glob Health. 2019;7:e721–34. Available at: https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(19)30080-4/abstract. Accessed 9 Oct 2020.
Morris SK, Awasthi S, Khera A, et al. Rotavirus mortality in India: estimates based on a nationally representative survey of diarrhoeal deaths. Bull World Health Organ. 2012;90:720–7.
Kang G, Arora R, Chitambar SD, et al. Multicenter, hospital-based surveillance of rotavirus disease and strains among indian children aged <5 years. J Infect Dis. 2009;200(Suppl 1):S147–53.
Kang G, Desai R, Arora R, et al. Diversity of circulating rotavirus strains in children hospitalized with diarrhea in India, 2005-2009. Vaccine. 2013;31:2879–83.
Mehendale S, Venkatasubramanian S, Girish Kumar CP, Kang G, Gupte MD, Arora R. Expanded Indian national rotavirus surveillance network in the context of rotavirus vaccine introduction. Indian Pediatr. 2016;53:575–81. Available at: http://link.springer.com/10.1007/s13312-016-0891-3. Accessed 22 Mar 2019.
National Rotavirus Surveillance Network, Kumar CPG, Venkatasubramanian S, Kang G, Arora R, Mehendale S. Profile and trends of rotavirus gastroenteritis in under 5 children in India, 2012–2014, preliminary report of the Indian National Rotavirus Surveillance Network. Indian Pediatr. 2016;53:619–22.
Malik A, Haldar P, Ray A, et al. Introducing rotavirus vaccine in the universal immunization programme in India: from evidence to policy to implementation. Vaccine. 2019;37:5817–24. Available at: http://www.sciencedirect.com/science/article/pii/S0264410X19310254. Accessed 15 Sep 2020.
Nair NP, Reddy NS, Giri S, et al. Rotavirus vaccine impact assessment surveillance in India: protocol and methods. BMJ Open [internet]. 2019;9:e024840. https://doi.org/10.1136/bmjopen-2018-024840.
Vesikari T, Van Damme P, Giaquinto C, et al. European Society for Paediatric Infectious Diseases consensus recommendations for rotavirus vaccination in Europe: update 2014. Pediatr Infect Dis J. 2015;34:635–43.
Babji S, Arumugam R, Sarvanabhavan A, et al. Multi-center surveillance of rotavirus diarrhea in hospitalized children <5 years of age in India, 2009-2012. Vaccine. 2014;32(Suppl 1):A10–2.
Giri S, Nair NP, Mathew A, et al. Rotavirus gastroenteritis in Indian children < 5 years hospitalized for diarrhoea, 2012 to 2016. BMC Public Health [Internet]. 2019;19:69. https://doi.org/10.1186/s12889-019-6406-0.
Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22.
Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33.
Rotavirus vaccines WHO position paper. January 2013 - Recommendations. Vaccine. 2013;31:6170–1.
Tate JE, Chitambar S, Esposito DH, et al. Disease and economic burden of rotavirus diarrhoea in India. Vaccine. 2009;27(Suppl 5):F18–24.
John J, Sarkar R, Muliyil J, Bhandari N, Bhan MK, Kang G. Rotavirus gastroenteritis in India, 2011-2013: revised estimates of disease burden and potential impact of vaccines. Vaccine. 2014;32(Suppl 1):A5–9.
Rheingans R, Anderson JD, Anderson B, Chakraborty P, Atherly D, Pindolia D. Estimated impact and cost-effectiveness of rotavirus vaccination in India: effects of geographic and economic disparities. Vaccine [Internet]. 2014;32:A140–50. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0264410X14007592. Accessed 19 Feb 2019.
Steele AD, Parashar UD. Rotavirus vaccines set to make inroads in Asia. Clin Infect Dis [Internet]. 2019;69:2071–3. Available at: https://academic.oup.com/cid/article/69/12/2071/5316811. Accessed 27 Aug 2020.
Burnett E, Parashar U, Tate J. Rotavirus vaccines: effectiveness, safety, and future directions. Pediatr Drugs. 2018;20:223–33.
Velázquez RF, Linhares AC, Muñoz S, et al. Efficacy, safety and effectiveness of licensed rotavirus vaccines: a systematic review and meta-analysis for Latin America and the Caribbean. BMC Pediatr [Internet]. 2017;17 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5237165/. Accessed 18 Feb 2019.
Lamberti LM, Ashraf S, Walker CLF, et al. A systematic review of the effect of rotavirus vaccination on diarrhoea outcomes among children younger than 5 years. Pediatr Infect Dis J. 2016;35:992–8.
Abou-Nader AJ, Sauer MA, Steele AD, et al. Global rotavirus vaccine introductions and coverage: 2006–2016. Hum Vaccin Immunother. 2018;14:2281–96 .
Burke RM, Tate JE, Kirkwood CD, et al. Current and new rotavirus vaccines. Curr Opin Infect Dis. 2019;32:435–44.
Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:2136–43.
Rose J, Homa L, Meropol SB, et al. Health impact and cost-effectiveness of a domestically-produced rotavirus vaccine in India: a model based analysis. PLoS One. 2017;12:e0187446.
Acknowledgements
The authors are grateful for the co-operation by all participants, parents/guardians and surveillance staff. They thank the team at Christian Medical College, Vellore for support, and the children and families for participation.
Funding
This work was supported by grants from the Bill and Melinda Gates Foundation to the Centers for Disease Control and Prevention, Atlanta, GA, USA (subcontract to Christian Medical College, Vellore grant no MOA#871-15SC) and the Translational Health Science and Technology Institute (grant no OPP1165083). The study was supported by the Indian Council of Medical Research, New Delhi; Ministry of Health and Family Welfare, Govt. of India and the State government of the participating institution.
Author information
Authors and Affiliations
Contributions
PD and GG conceived the paper and obtained approvals for the project. NPN and VT did the analysis of the data. PD wrote the first draft, GG and JS revised the draft. All authors read, critically revised, and approved final manuscript. GG will act as guarantor for this paper.
Corresponding author
Ethics declarations
Ethics Approval
The project was approved by Institutional Ethics Committee of Pt. B. D. Sharma University of Health Sciences, Rohtak, Haryana (India).
Conflict of Interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dalal, P., Gathwala, G., Singh, J. et al. Gastroenteritis in Haryana, India Post Introduction of Rotavirus Vaccine. Indian J Pediatr 88 (Suppl 1), 10–15 (2021). https://doi.org/10.1007/s12098-020-03614-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-020-03614-3