Abstract
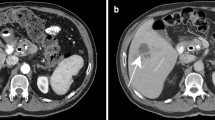
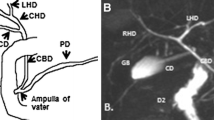
Hyperbilirubinemia is a common occurrence in neonates; it may be physiological or pathological. Conjugated hyperbilirubinemia may result from medical or surgical causes, and can result in irreversible liver damage if untreated. The aim of imaging is the timely diagnosis of surgical conditions like biliary atresia and choledochal cysts. Abdominal ultrasound is the first line imaging modality, and Magnetic resonance cholangiopancreatography (MRCP) also has a role, especially in pre-operative assessment of choledochal cysts (CDCs). For biliary atresia, the triangular cord sign and gallbladder abnormalities are the two most useful ultrasound features, with a combined sensitivity of 95%. Liver biopsy has an important role in pre-operative evaluation; however, the gold standard for diagnosis of biliary atresia remains an intra-operative cholangiogram. Choledochal cysts are classified into types according to the number, location, extent and morphology of the areas of cystic dilatation. They are often associated with an abnormal pancreaticobiliary junction, which is best assessed on MRCP. Caroli’s disease or type 5 CDC comprises of multiple intrahepatic cysts. CDCs, though benign, require surgery as they may be associated with complications like cholelithiasis, cholangitis and development of malignancy. Severe unconjugated hyperbilirubinemia puts neonates at high risk of developing bilirubin induced brain injury, which may be acute or chronic. Magnetic resonance imaging of the brain is the preferred modality for evaluation, and shows characteristic involvement of the globus pallidi, subthalamic nuclei and cerebellum – in acute cases, these areas show T1 hyperintensity, while chronic cases typically show hyperintensity on T2 weighted images.










Similar content being viewed by others
References
Bhatia V, Bavdekar A, Matthai J, Waikar Y, Sibal A. Management of neonatal cholestasis: consensus statement of the pediatric gastroenterology chapter of Indian academy of pediatrics. Indian Pediatr. 2014;51:203–10.
Poddar U, Thapa BR, Das A, Bhattacharya A, Rao KLN, Singh K. Neonatal cholestasis: differentiation of biliary atresia from neonatal hepatitis in a developing country. Acta Paediatr. 2009;98:1260–64.
Rastogi A, Krishnani N, Yachha SK, Khanna V, Poddar U, Lal R. Histopathological features and accuracy for diagnosing biliary atresia by prelaparotomy liver biopsy in developing countries. J Gastroenterol Hepatol. 2009;24:97–102.
Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the north American Society for Pediatric Gastroenterology, hepatology, and nutrition and the European Society for Pediatric Gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr. 2017;64:154–68.
De Bruyne R, Van Biervliet S, Vande Velde S, Van Winckel M. Clinical practice: neonatal cholestasis. Eur J Pediatr. 2011;170:279–84.
Serinet M-O, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics. 2009;123:1280–6.
Asai A, Miethke A, Bezerra JA. Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes. Nat Rev Gastroenterol Hepatol. 2015;12:342–52.
Zhou L, Shan Q, Tian W, Wang Z, Liang J, Xie X. Ultrasound for the diagnosis of biliary atresia: a meta-analysis. Am J Roentgenol. 2016;206:W73–82.
Choi SO, Park WH, Lee HJ, Woo SK. “Triangular cord”: a sonographic finding applicable in the diagnosis of biliary atresia. J Pediatr Surg. 1996;31:363–6.
Lee H-J, Lee S-M, Park W-H, Choi S-O. Objective criteria of triangular cord sign in biliary atresia on US scans. Radiology. 2003;229:395–400.
Zhou L-Y, Wang W, Shan Q, et al. Optimizing the US diagnosis of biliary atresia with a modified triangular cord thickness and gallbladder classification. Radiology. 2015;277:181–91.
Humphrey TM, Stringer MD. Biliary atresia: US diagnosis. Radiology. 2007;244:845–51.
Aziz S, Wild Y, Rosenthal P, Goldstein RB. Pseudo gallbladder sign in biliary atresia—an imaging pitfall. Pediatr Radiol. 2011;41:620–6.
Tan Kendrick APA, Phua KB, Ooi BC, Tan CEL. Biliary atresia: making the diagnosis by the gallbladder ghost triad. Pediatr Radiol. 2003;33:311–5.
Jawaheer G, Pierro A, Lloyd DA, Shaw NJ. Gall bladder contractility in neonates: effects of parenteral and enteral feeding. Arch Dis Child Fetal Neonatal Ed. 1995;72:F200–2.
Ikeda S, Sera Y, Akagi M. Serial ultrasonic examination to differentiate biliary atresia from neonatal hepatitis--special reference to changes in size of the gallbladder. Eur J Pediatr. 1989;148:396–400.
Kim WS, Cheon J-E, Youn BJ, et al. Hepatic arterial diameter measured with US: adjunct for US diagnosis of biliary atresia. Radiology. 2007;245:549–55.
Lee MS, Kim M-J, Lee M-J, et al. Biliary atresia: color doppler US findings in neonates and infants. Radiology. 2009;252:282–9.
Koob M, Pariente D, Habes D, Ducot B, Adamsbaum C, Franchi-Abella S. The porta hepatis microcyst: an additional sonographic sign for the diagnosis of biliary atresia. Eur Radiol. 2017;27:1812–21.
Norton KI, Glass RB, Kogan D, Lee JS, Emre S, Shneider BL. MR cholangiography in the evaluation of neonatal cholestasis: initial results. Radiology. 2002;222:687–91.
Kim YH, Kim M-J, Shin HJ, et al. MRI-based decision tree model for diagnosis of biliary atresia. Eur Radiol. 2018;28:3422–31.
Sung S, Jeon TY, Yoo S-Y, et al. Incremental value of MR cholangiopancreatography in diagnosis of biliary atresia. PLoS One. 2016;11:e0158132.
Liu B, Cai J, Xu Y, et al. Three-dimensional magnetic resonance cholangiopancreatography for the diagnosis of biliary atresia in infants and neonates. PLoS One. 2014;9:e88268.
Caponcelli E, Knisely AS, Davenport M. Cystic biliary atresia: an etiologic and prognostic subgroup. J Pediatr Surg. 2008;43:1619–24.
Zhou L-Y, Guan B-Y, Li L, et al. Objective differential characteristics of cystic biliary atresia and choledochal cysts in neonates and young infants. J Ultrasound Med. 2012;31:833–41.
Han S, Jeon TY, Hwang SM, et al. Imaging findings of Alagille syndrome in young infants: differentiation from biliary atresia. Br J Radiol. 2017;90:20170406.
Edil BH, Olino K, Cameron JL. The current management of choledochal cysts. Adv Surg. 2009;43:221–32.
Miyano T, Yamataka A. Choledochal cysts. Curr Opin Pediatr. 1997;9:283–8.
Alonso-Lej F, Rever WB, Pessagno DJ. Congenital choledochal cyst, with a report of 2, and an analysis of 94, cases. Int Abstr Surg. 1959;108:1–30.
Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263–9.
Todani T. Congenital choledochal dilatation: classification, clinical features, and long-term results. J Hepato-Biliary-Pancreat Surg. 1997;4:276–82.
Lewis VA, Adam SZ, Nikolaidis P, et al. Imaging of choledochal cysts. Abdom Imaging. 2015;40:1567–80.
Lee HK, Park SJ, Yi BH, Lee AL, Moon JH, Chang YW. Imaging features of adult choledochal cysts: a pictorial review. Korean J Radiol. 2009;10:71–80.
Singham J, Yoshida EM, Scudamore CH. Choledochal cysts: part 1 of 3: classification and pathogenesis. Can J Surg. 2009;52:434–40.
Liu Q-Y, Lai D-M, Gao M, et al. MRI manifestations of adult choledochal cysts associated with biliary malignancy: a report of ten cases. Abdom Imaging. 2013;38:1061–70.
Ono S, Fumino S, Shimadera S, Iwai N. Long-term outcomes after hepaticojejunostomy for choledochal cyst: a 10- to 27-year follow-up. J Pediatr Surg. 2010;45:376–8.
Bhutani VK, Zipursky A, Blencowe H, et al. Neonatal hyperbilirubinemia and rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res. 2013;74:86–100.
Watchko JF. Bilirubin-induced neurotoxicity in the preterm neonate. Clin Perinatol. 2016;43:297–311.
Wisnowski JL, Panigrahy A, Painter MJ, Watchko JF. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422–8.
Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage--mechanisms and management approaches. N Engl J Med. 2013;369:2021–30.
Shroff MM, Soares-Fernandes JP, Whyte H, Raybaud C. MR imaging for diagnostic evaluation of encephalopathy in the newborn. Radiographics. 2010;30:763–80.
Sarı S, Yavuz A, Batur A, Bora A, Caksen H. Brain magnetic resonance imaging and magnetic resonance spectroscopy findings of children with kernicterus. Pol J Radiol. 2015;80:72–80.
Oakden WK, Moore AM, Blaser S, Noseworthy MD. 1H MR spectroscopic characteristics of kernicterus: a possible metabolic signature. Am J Neuroradiol. 2005;26:1571–4.
Okumura A, Hayakawa F, Maruyama K, Kubota T, Kato K, Watanabe K. Single photon emission computed tomography and serial MRI in preterm infants with kernicterus. Brain Dev. 2006;28:348–52.
Gkoltsiou K, Tzoufi M, Counsell S, Rutherford M, Cowan F. Serial brain MRI and ultrasound findings: relation to gestational age, bilirubin level, neonatal neurologic status and neurodevelopmental outcome in infants at risk of kernicterus. Early Hum Dev. 2008;84:829–38.
Acknowledgement
The authors acknowledge Dr. Rama Anand and Dr. Suvasini Sharma for help with the images.
Author information
Authors and Affiliations
Contributions
All three authors contributed to the Manuscript preparation and editing. Dr. Rama Anand, Director-Professor and HOD, Department of Radio-Diagnosis, Lady Hardinge Medical College will act as guarantor for this paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbey, P., Kandasamy, D. & Naranje, P. Neonatal Jaundice. Indian J Pediatr 86, 830–841 (2019). https://doi.org/10.1007/s12098-019-02856-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-019-02856-0