Abstract
Objective
To compare the effectiveness of nasal continuous positive airway pressure (NCPAP) cycling with continuous NCPAP in the successful weaning of preterm infants of 250–286 wk gestation to nasal prongs.
Methods
A total of 30 infants with a gestational age (GA) of 250–286 wk, ventilated for respiratory distress syndrome (RDS) and extubated to NCPAP were eligible for the study. They were randomized to NCPAP cycling [Group A: cycling between NCPAP of 4 cm and 1 liter per minute (LPM) of nasal prongs] or to continuous NCPAP at 4 cm of H2O (Group B). Primary outcome was successful weaning off NCPAP to nasal prongs at the end of 72 h of the intervention and remaining off NCPAP for the next 72 h.
Results
The demographic characteristics were similar in both the groups. Infants were randomized to Group A (n = 13) and Group B (n = 17). The primary outcome was not significantly different between the groups (successful weaning to nasal prongs: 31 vs. 41 %; p 0.71).
Conclusions
In this pilot, feasibility study there were no differences in the rates of successful weaning of NCPAP to nasal prongs using either cycling NCPAP or continuous NCPAP in preterm infants. A need exists for a large randomized controlled trial (RCT) to determine the role of cycling NCPAP on neonatal outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasal continuous positive airway pressure (NCPAP) is being extensively used in preterm infants both as a primary mode of respiratory support and as post extubation support. NCPAP exerts its beneficial respiratory effects mainly by a reduction in the physiological dead space; a decrease in apneic episodes by splinting the airways; a decrease in hypoxic episodes by maintaining the functional residual capacity; and, a decrease in airway resistance [1–4]. The other physiological effects of NCPAP include improved lung compliance, lung growth, elastic work of breathing, ventilation-perfusion ratio and stabilization of chest wall [5–9].
Prolonged use of mechanical ventilation is associated with infections, subglottic stenosis and aspiration [10–12]. Therefore, clinicians strive to wean early from the ventilator and avoid reintubation once infants are extubated. NCPAP has been used to prevent extubation failure and as an alternative to intubation and ventilation for respiratory distress syndrome in very preterm infants. Early discontinuation of NCPAP carries the risk of pulmonary atelectasis, apnea and bradycardia. The most common reasons for failure to wean on NCPAP are respiratory acidosis, apneas, bradycardias, and increasing oxygen requirements. In a prospective study by Abdel-Hady et al. on preterm infants with resolving respiratory distress syndrome, NCPAP impeded systemic and pulmonary venous return but did not compromise systemic arterial pressure or heart rate [13].
NCPAP is commonly used after extubation. However, there is uncertainty regarding the optimal approach to wean NCPAP. Some of the techniques used include decreasing the pressure, weaning the “time off” NCPAP and abrupt discontinuation of NCPAP irrespective of the level of distending pressure [14, 15]. There is equipoise regarding the role of cycling NCPAP with continuous nasal flow (CNF) vs. continuous NCPAP without CNF in premature infants. The authors speculate that NCPAP cycling will gradually shift the work of breathing from the supportive intervention to the baby without producing fatigue of respiratory muscles. Currently, both these modes of weaning are being used in authors’ NICU. It is largely determined by the personal preferences of neonatologists. Therefore, it would be prudent to study the benefits of NCPAP cycling vs. continuous NCPAP in premature infants. In a randomized controlled trial of discontinuation of NCPAP, Abdel-Hady found that most preterm infants breathing room air, tolerated a 6-h pause in NCPAP with no increase in apnea and bradycardia [14]. A reduction in the subsequent use of NCPAP was also observed. Findings from Abdel-Hady’s study led to the development of a strategy to further reduce ventilator induced trauma and thus bronchopulmonary dysplasia (BPD) [14]. This strategy used non-invasive intermittent continuous positive airway pressure ventilation by alternating NCPAP with continuous nasal flow via nasal prongs for extremely low birth weight (ELBW) infants [14]. This method of ventilation is termed cycling of NCPAP. Although the mode of delivering NCPAP has been extensively studied, there is no consensus regarding how to wean from NCPAP. In a recent study, Singh et al. compared the weaning strategy of either gradual reduction of NCPAP (pressure) or increasing duration of “time off” NCPAP in very low birth weight infants [16]. This study suggested that gradual reduction in NCPAP pressure may accelerate prompt weaning compared with periods of increasing time spent off NCPAP [16]. Soe’s study showed that NCPAP weaning can be successful by either method [17]. However, in preterm infants born between 24 to 27 wk gestation, pressure weaning may be more appropriate [17].
The objective of this pilot study was to compare the effectiveness of NCPAP cycling with continuous NCPAP in successful weaning of preterm infants of 250–286 wk gestation to nasal prongs.
Material and Methods
This prospective, open label, pilot, feasibility, randomized controlled trial study was conducted between Jan 2011 and Dec 2011 in the regional tertiary level Neonatal Intensive Care Unit (NICU) at Foothills Medical Centre, Calgary. Preterm infants between 250–286 wk gestation, ventilated for respiratory distress syndrome (RDS) and extubated to NCPAP for at least 72 h were eligible for inclusion. Infants with major congenital and chromosomal anomalies were excluded. The institutional ethics review board of the University of Calgary approved this study. Signed consent was obtained from the parents of all study participants. The trial was registered with clinical trial.gov (NCT02114112).
Randomization was achieved using a computerized random number generator, using block sizes of 2 and 4 to increase the likelihood of equal enrolment into each group. Randomization was done on site, concealed and undertaken by health personnel not involved in the study. Treatment allocation cards were kept in opaque sequentially numbered sealed envelopes that were kept in a locked drawer in NICU. Envelopes were opened sequentially at the time of allocation.
Infants who met the inclusion criteria were identified and informed written consent was obtained from the parents or the legal guardian. All the infants were extubated to Infant Flow Driver NCPAP/Bi-level positive airway pressure (BiPAP) as per the unit practice. A starting NCPAP pressure of 6–8 cm H2O was used as per unit policy. NCPAP pressures were weaned when an infant required FiO2 < 25 %. Infants who remained on NCPAP for at least 72 h and who were weaned to NCPAP of 4 cm H2O were randomized to either of two groups [Group A (Cycling group) and Group B (Continuous NCPAP group)]. Infants in Group A were cycled between NCPAP and nasal prongs. For the first 12 h, infants received 10 h of NCPAP and 2 h of 1LPM of nasal prongs (NP). For the next 12 h, infants received 8 h of NCPAP and 4 h of NP. For the subsequent 24 h, infants alternated between 6 h of NCPAP and 6 h of NP. For the last 24 h of intervention they alternated between 4 h of NCPAP and 8 h of NP. Infants randomized to group B received continuous NCPAP at a distending pressure of 4 cm H2O for 72 h. Figure 1 depicts the interventions in both the arms. Both the groups, after 72 h of intervention, were weaned to 1LPM NP. 1LPM NP was chosen as this was the standard practice in authors’ NICU based on the definition of low flow NP by the Canadian Neonatal Network, which defines low flow nasal prongs as less than 1.5LPM. During the study period in the NICU, heated humidified high flow-nasal cannula was used when the flow rate increased above 1 L/min particularly in babies >28 wk of GA. During the intervention period, all infants were scored using the ACoRN respiratory score on a 12 hourly basis. The need for blood gas assessment and chest X-ray was left to the discretion of the responsible physician. In the authors’ NICU oxygen saturation limits between 88 to 92 % are targeted for preterm infants <32 wk.
Successful weaning was defined when an infant continued to be on 1LPM NP for at least 72 h. Weaning was considered unsuccessful if: (i) ACoRN respiratory score ≥5 or increase from previous score by 2 points in a 12 h period or (ii) supplemental FiO2 > 0.30 or (iii) acidosis with pH < 7.25 or (iv) apnea with bradycardia or desaturation requiring stimulation >1/h or (v) apneic episode requiring Positive Pressure Ventilation (PPV) with Bag & Mask in a 12 h period [18].
The primary outcome was the number of babies who came off NCPAP at the end of 72 h of the intervention and remained off NCPAP for 72 h. Secondary outcomes were total duration of NCPAP in days, total duration of mechanical ventilation after reintubation in days, BPD at 36 wk of postmenstrual age (oxygen requirement at 36 wk of PMA) [19] and ROP stage 3 or more [20].
Since this was a pilot, feasibility study, the authors planned for a convenience sample of 40 with 20 infants in each arm. The study was terminated after the enrollment of 30 infants after introduction of a Quality initiative respiratory bundle, which protocolized respiratory management in preterm infants. Data was analyzed using statistical package SAS Version 9.3 (SAS Institute Inc., Cary, NC). For categorical variables, Fisher’s exact test was used to compare differences in proportion, and the risk difference with 95 % confidence intervals was reported. As the data for continuous variables were not normally distributed, the Mann-Whitney U test was used to compare the two groups, with the Hodges-Lehmann median difference and 95 % confidence intervals reported.
Results
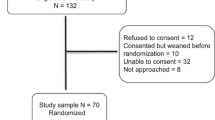
Thirty infants were enrolled; 13 in-Group A (NCPAP cycling) and 17 in-Group B (continuous NCPAP). The study population was described as per consort diagram in Fig. 2. Table 1 depicts the baseline characteristics of the infants in both groups. The proportions of male infants were higher in group B (71 %) as compared to group A (31 %). Median age at study entry was 6 d (IQR: 6–10) in group A as compared to 16 d (IQR: 6–26) in group B. Median duration of ventilation prior to the intervention was higher in group B [7 (5–18) vs. 36 (8–58)].
The results of primary and secondary outcomes are shown in Table 2. In NCPAP cycling group, 31 % of infants were successfully weaned to NP compared to 41 % in the continuous NCPAP group (P 0.71). The median NCPAP duration was longer in the cycling group despite shorter duration of ventilation compared with NCPAP group. The rates of BPD were higher in cycling group when compared to continuous NCPAP group (62 vs. 35 %) although the difference was not statistically significant. Other neonatal outcomes of severe intraventricular hemorrhage (IVH grade ≥3), Patent ductus arteriosus (PDA) and late onset sepsis (LOS, culture positivity in blood or CSF after 72 h of life) were similar in both the groups.
Discussion
The present study did not demonstrate significant differences between the two methods of weaning from NCPAP. Also, there were no differences in the incidences of BPD and retinopathy of prematurity (ROP) between the two groups.
NCPAP is being extensively used in NICUs as it is non-invasive and has beneficial effects in the preterm neonates. There is no consensus on how to wean the infants from NCPAP although various studies have explored this [14–17, 21–26]. In a survey conducted among Australian and New Zealand neonatologists, the practice of weaning CPAP varied. Around 48 % of neonatologists used cycling CPAP and 50 % used gradual weaning of the pressures [15].
A study by Heygi et al. demonstrated that infants tolerated abrupt withdrawal of NCPAP from distending pressures of 6 cm H2O and that decreasing the pressures may not be required [22]. However the infants in this study were of higher gestation (29 to 38 wk gestation) as compared to the present study.
In the index study, authors did not find any significant difference in outcomes between the two methods of weaning from NCPAP. However, Soe et al. showed that pressure weaning was more successful than time weaning in infants between 24–27 wk although there was no difference in the success rates in infants between 28–31 wk [17]. Singh et al. in 2006 reported that weaning the pressures on NCPAP facilitated faster respiratory weaning in <1500 g compared to NCPAP cycling with low flow NP [16]. In the same study, median duration of weaning via pressure reduction was 1.5 d vs. 8.9 d in the time off or cycling group (p < 0.001). This study also revealed that median time spent on NCPAP was proportionately shorter in the pressure group compared to time off cycling group [6.0 (2.1–60.0)d vs. 13.2 (10.0–46.0)] (p 0.001). The present finding of the median NCPAP duration being longer in the cycling group compared to NCPAP group despite shorter duration of mechanical ventilation is consistent with Singh’s study [16].
Singh and Soe’s studies [16, 17] differed from the present study with respect to the duration of weaning in that they used 7 d for the weaning period compared to 72 h in the index study. In 2010, a retrospective cohort study of infants <32 wk demonstrated longer duration of CPAP and hospital stay with non-cycling CPAP when compared to CPAP cycling [23].
The present study included infants born between 250–286 wk gestational age. In the index study all babies received caffeine before extubation and stayed on CPAP of 4 at least for 72 h before randomly allocating to one of the interventions. In a multicenter RCT by Todd et al., three different modes of NCPAP weaning (abruptly stopping the NCPAP, NCPAP cycling with unsupported off periods and, NCPAP cycling with off periods supported by 0.5LPM) were studied in infants <30 wk gestation. Infants in whom NCPAP was abruptly discontinued, the time to wean NCPAP and the CPAP duration were significantly shorter [25].
In a pilot RCT there was no difference in CPAP weaning success rates between sudden (non-cycling CPAP) and gradual weaning (cycling CPAP) in infants <32 wk [24]. The methodology of this study was different from the index pilot study as the positive end-expiratory pressure (PEEP) during the weaning process was 5 cm (the authors used a pressure of 4 cm) and the duration of weaning process was 7 d.
In a recent review in 2011, authors concluded that gradual weaning and discontinuation is associated with less time on NCPAP, shorter duration of oxygen therapy and hospital stay [27]. This review included only three studies [15, 16, 25].
Infant flow Driver CPAP with either mask or prongs is used as an interface in authors’ NICU. All infants in the present study received the same predetermined distending pressure of 4 cm H2O before the intervention was started unlike many of the studies mentioned above. The median duration of CPAP was 7 d higher in the cycling group but did not reach statistical significance probably because of the small sample size. In the index study there was a higher rate of BPD in the cycling group compared to continuous NCPAP (62 vs. 35 %, p 0.88) although it was not statistically significant and could again be explained by the small sample size.
There are a few limitations to the present study. Blinding was not feasible because of the nature of intervention used. The sample size was small as it was a pilot feasibility study. The authors had decided upon a sample size of 40 prior to the implementation of the study. However the study was prematurely stopped due to the introduction of a respiratory bundle quality initiative in authors’ unit with the aim to reduce BPD rates. The number of male infants, the median age of entry into study and the median duration of ventilation prior to the intervention were higher in the continuous NCPAP group; whether this difference plays a role in successful weaning from NCPAP is unknown. The rates of BPD clinically differed among the groups although they did not reach statistical significance probably because of the small sample size.
Conclusions
In this pilot, feasibility study comparing NCPAP cycling to continuous NCPAP for weaning preterm infants from NCPAP, authors did not find any significant difference in rates of successful weaning between the two groups. Larger RCTs are needed to determine the best weaning modes from NCPAP for preterm infants and their impact on neonatal outcomes.
Abbreviations
- BiPAP:
-
Bi-level positive airway pressure
- BPD:
-
Bronchopulmonary dysplasia
- NCPAP:
-
Nasal continuous positive airway pressure
- NP:
-
Nasal prongs
- RDS:
-
Respiratory distress syndrome
- ROP:
-
Retinopathy of prematurity
References
Bancalari E, Claure N. The evidence for non-invasive ventilation in the preterm infant. Arch Dis Child Fetal Neonatal Ed. 2013;98:F98–102.
Chowdhury O, Wedderburn CJ, Duffy D, Greenough A. CPAP review. Eur J Pediatr. 2012;171:1441–8.
Davis PG, Morley CJ, Owen LS. Non-invasive respiratory support of preterm neonates with respiratory distress: continuous positive airway pressure and nasal intermittent positive pressure ventilation. Semin Fetal Neonatal Med. 2009;14:14–20.
Morley C. Continuous distending pressure. Arch Dis Child Fetal Neonatal Ed. 1999;81:F152–6.
Diblasi RM. Nasal continuous positive airway pressure (CPAP) for the respiratory care of the newborn infant. Respir Care. 2009;54:1209–35.
Locke R, Greenspan JS, Shaffer TH, Rubenstein SD, Wolfson MR. Effect of nasal CPAP on thoracoabdominal motion in neonates with respiratory insufficiency. Pediatr Pulmonol. 1991;11:259–64.
Richardson CP, Jung AL. Effects of continuous positive airway pressure on pulmonary function and blood gases of infants with respiratory distress syndrome. Pediatr Res. 1978;12:771–4.
Roehr CC, Proquitte H, Hammer H, Wauer RR, Morley CJ, Schmalisch G. Positive effects of early continuous positive airway pressure on pulmonary function in extremely premature infants: results of a subgroup analysis of the COIN trial. Arch Dis Child Fetal Neonatal Ed. 2011;96:F371–3.
Zhang S, Garbutt V, McBride JT. Strain-induced growth of the immature lung. J Appl Physiol (1985). 1996;81:1471–6.
Avery ME, Tooley WH, Keller JB, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics. 1987;79:26–30.
Fan LL, Flynn JW, Pathak DR. Risk factors predicting laryngeal injury in intubated neonates. Crit Care Med. 1983;11:431–3.
Goodwin SR, Graves SA, Haberkern CM. Aspiration in intubated premature infants. Pediatrics. 1985;75:85–8.
Abdel-Hady H, Matter M, Hammad A, El-Refaay A, Aly H. Hemodynamic changes during weaning from nasal continuous positive airway pressure. Pediatrics. 2008;122:e1086–90.
Abdel-Hady H, Mohareb S, Khashaba M, Abu-Alkhair M, Greisen G. Randomized controlled trial of discontinuation of nasal-CPAP in stable preterm infants breathing room air. Acta Paediatr. 1998;87:82–7.
Jardine L, Davies MW. Withdrawal of neonatal continuous positive airway pressure: current practice in Australia. Pediatr Int. 2008;50:572–5.
Singh SD, Bowe L, Clarke P, et al; European Academy of Pediatrics, Book of Abstracts. Is decreasing pressure or increasing time off the better strategy in weaning VLBW infants from nasal CPAP? Eur J Pediatr. 2006;165:S48.
Soe A, Hodgkinson J, Jani B, Ducker DA, European Academy of Pediatrics, Book of Abstracts. Nasal continuous positive airway pressure weaning in preterm infants. Eur J Pediatr. 2006;165:S48–9.
Solimano A, Ling E, O’Flaherty D, et al. Acute care of at-risk newborns: a resource and learning tool for healthcare professionals. Vancouver, BC, Canada: The ACoRN Editorial Board; 2005.
Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9.
International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
Abdel-Hady H, Shouman B, Aly H. Early weaning from CPAP to high flow nasal cannula in preterm infants is associated with prolonged oxygen requirement: a randomized controlled trial. Early Hum Dev. 2011;87:205–8.
Hegyi T, Hiatt IM. Discontinuation of continuous positive airways pressure in infants with respiratory distress syndrome. Arch Dis Child. 1979;54:722–4.
Mohamed A, Diambomba Y, Deshpande P, et al. Does approach of weaning from CPAP influence neonatal outcomes in preterm infants? E-PAS. 2010:1472.218.
Rastogi S, Wong W, Gupta A, Bhutada A, Deepa R, Maimonides NG. Gradual versus sudden weaning from nasal CPAP in preterm infants: a pilot randomized controlled trial. Respir Care. 2013;58:511–6.
Todd DA, Wright A, Broom M, et al. Methods of weaning preterm babies <30 weeks gestation off CPAP: a multicentre randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2012;97:F236–40.
Wong W, Rajasekhar H, Rastogi D, Gupta A, Bhutada A, Rastogi S. Prospective randomized control trial of weaning from CPAP in VLBW babies. E-PAS. 2010:4407.338.
Jardine LA, Inglis GD, Davies MW. Strategies for the withdrawal of nasal continuous positive airway pressure (NCPAP) in preterm infants. Cochrane Database Syst Rev. 2011;2:CD006979.
Acknowledgements
The authors would like to acknowledge Dr Sue Ross, PhD Epidemiology for designing and planning the randomization methodology.
Contributions
VN, KS and AL primarily designed the protocol and framework of the study. HA, YR, AH, AA, KO and MK contributed to the development of the protocol. VN and KS participated in patient recruitment. ST performed statistical analysis. VN and AL had primary responsibility for writing the manuscript. All the authors read, commented and approved the final manuscript. AL will act as guarantor for this paper.
Conflict of Interest
None.
Source of Funding
Alberta Children’s Hospital Research Institute, University of Calgary, Calgary, Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial Registration: ClinicalTrials.gov NCT02114112
Rights and permissions
About this article
Cite this article
Nair, V., Swarnam, K., Rabi, Y. et al. Effect of Nasal Continuous Positive Airway Pressure (NCPAP) Cycling and Continuous NCPAP on Successful Weaning: A Randomized Controlled Trial. Indian J Pediatr 82, 787–793 (2015). https://doi.org/10.1007/s12098-015-1721-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-015-1721-7