Abstract
Purpose
HER2 overexpression in breast cancer correlates with poor outcomes. The incorporation of Trastuzumab into the treatment regimen has notably improved patient prognoses. However, cardiotoxicity emerges in approximately 20% of patients treated with the drug. This study aims to investigate the association between the HER2 655 A > G polymorphism, Trastuzumab-induced cardiotoxicity, and patient survival.
Methods
The study involved 88 patients treated with Trastuzumab. Cardiotoxicity, defined as a reduction in left ventricular ejection fraction (LVEF) from baseline or the emergence of clinical signs of congestive heart failure, was identified during treatment follow-up. Genotyping of HER2 655 A > G employed TaqMan SNP technology.
Results
Genotype frequencies of HER2/neu 655 (53 AA, 32 AG, and 3 GG) were consistent with Hardy–Weinberg equilibrium. No significant differences were observed in mean baseline LVEF between patients who developed cardiotoxicity and those who did not. Within these groups, neither AA nor AG genotypes showed an association with changes in mean baseline or reduced LVEF levels. Logistic regression analysis, adjusted for hormonal status and anthracycline treatment, revealed that AG genotype carriers face a significantly higher risk of cardiotoxicity compared to AA carriers (OR = 4.42; p = 0.037). No association was found between the HER2/neu 655 A > G polymorphism and disease-free or overall survival, regardless of whether the data was adjusted for stage or not.
Conclusion
HER2 655 A > G polymorphism is significantly linked to an increased risk of Trastuzumab-induced cardiotoxicity but does not correlate with variations in disease-free survival or overall survival rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human epidermal growth factor receptor 2 (HER2) is a proto-oncogene that encodes a transmembrane protein with tyrosine kinase activity. Approximately 20% of breast cancer patients exhibit overexpression of HER2, which is associated with high rates of recurrence and poor prognoses [1, 2].
Activation of HER2 proteins leads to phosphorylation events, initiating various signaling pathways. These pathways result in the inactivation of proteins that induce apoptosis and up-regulate genes responsible for cellular growth, thereby facilitating tumor cell proliferation and the progression of breast cancer [1].
Trastuzumab, a humanized monoclonal antibody, selectively binds to the extracellular domain of HER2 [3]. Its administration yields an anti-proliferative effect on HER2-positive cells [4] and has been shown to significantly increase response rates in patients with HER2-positive breast cancer, reducing mortality, recurrence, and metastasis rates, and improving overall survival [5,6,7]. However, a portion of patients develop cardiotoxicity from anti-HER2 therapies [6,7,8,9], particularly when Trastuzumab is used in combination with anthracycline-based chemotherapy [4, 5, 10]. The risk of cardiac events induced by Trastuzumab has been reported to range from 3 to 7% when administered alone [11] and as high as 27% when combined with anthracycline [11,12,13].
The potential of Trastuzumab to cause cardiac complications is linked to its interference with the neuregulin-1 mediated signaling pathway, an essential growth factor for protecting and maintaining cardiac function under oxidative stress. It activates the HER4 receptor, which then forms a dimer with HER2 [14], promoting cardiomyocyte survival and the organization of cardiac contractile structures [15]. Trastuzumab may disrupt the heart ability to protect itself from reactive oxygen species (ROS) and regulate programmed cardiomyocyte death [14, 16].
Given the potential cardiotoxic effects of Trastuzumab, patients necessitate careful monitoring of left ventricular (LV) function, typically evaluated by measuring the LV ejection fraction (LVEF) [17]. However, LVEF may not detect early subtle changes and generally reflects advanced myocyte damage when reduced [18,19,20,21].
Several genetic polymorphisms in the HER2 gene have been identified [22, 23]. Notably, a polymorphism involves a nucleotide change in the coding region of the transmembrane domain at codon 655, where an adenine (A) is replaced by a guanine (G). This substitution alters the protein structure, replacing isoleucine (Ile) (encoded by ATC) with valine (Val) (encoded by GTC) in the transmembrane domain [24]. The presence of the 655 A variant in the HER2 transmembrane domain may affect the dimerization capability of active HER2 proteins with other HER family members, potentially leading to reduced signal transduction compared to the 655 G variant [23]. Epidemiological studies suggest a weak association between the presence of the 655Val (G) allele and an increased risk of breast cancer in Caucasian populations, while in African women, the Val/Val (G/G) genotype is linked to a higher risk of breast cancer [25]. The 655 A > G polymorphism has also been implicated in influencing the response to Trastuzumab treatment and the likelihood of experiencing cardiac adverse effects [26]. The presence of the G allele may render cardiomyocytes more dependent on HER2 signaling and sensitive to Trastuzumab [27] (Fig. 1).
The objectives of the study include investigating the association between the 655 A > G polymorphism and Trastuzumab-induced cardiotoxicity, as well as its correlation with survival in breast cancer patients. Given the potential of this polymorphism to influence the sensitivity of tumor cells to Trastuzumab, it may have an impact on the survival of breast cancer patients. Understanding the role of the 655 A > G polymorphism in both cardiotoxicity risks and patient survival is crucial for the development of personalized treatment strategies in HER2-positive breast cancer.
Materials and methods
Patients and treatment
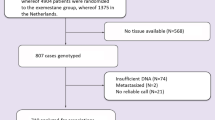
Eighty-eight HER2-positive early-stage breast cancer patients undergoing Trastuzumab therapy were recruited from San Cecilio University Hospital in Granada (Spain). Informed, written consent was obtained from all participants, and the study received approval from the Provincial Ethical Committee of Granada. Trastuzumab was administered intravenously with an initial dose of 8 mg/kg, followed by a maintenance dose of 6 mg/kg every three weeks, or with an initial dose of 4 mg/kg, followed by a maintenance dose of 2 mg/kg on weekly schedules. Trastuzumab was also administered subcutaneously at a fixed dose of 600 mg. In addition to trastuzumab treatment, fifty-seven patients out of the total received combined therapy with both anthracyclines and taxanes, whereas fifteen patients received exclusive anthracycline therapy, and three patients received exclusive taxane therapy.
Cardiac toxicity
Cardiac function was assessed in each patient through the measurement of LVEF and clinical examination to detect congestive heart failure or sustained asymptomatic subclinical cardiac toxicity. The patients were assessed through echocardiography or isotopic ventriculography. Patients initially evaluated by one methodology continued their evaluation using the same method throughout the study. LVEF measurements were conducted before the initiation of Trastuzumab treatment and then at 3, 6, 9, and 12 months post the initial administration. Trastuzumab treatment was initiated if LVEF was above 55%, or between 50 and 55% with cardiologist approval. Cardiac toxicity during treatment was defined as any of the following: a decrease of at least 10% from baseline with a resulting LVEF of less than 50% at follow-up, a decrease of 15% relative to baseline LVEF, any decrease resulting in an LVEF of less than 45% at least once during treatment, or clinical manifestation of congestive heart failure [26, 28, 29].
HER2 655 A > G genotyping
For genotyping, cellular DNA was isolated from saliva samples using standard procedures. The HER2 655 A > G (Ile/Val) (rs1136201) gene promoter single-nucleotide polymorphism was genotyped using the TaqMan SNP Genotyping Assay technology (Life Technologies, Foster City, California, USA). Genotyping analysis was performed using the Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems Inc., Foster City, California, USA) and SDS 2.0.4 software (Applied Biosystems Inc.).
Statistical analysis
The frequency distribution of HER2 655 A > G polymorphisms was tested for Hardy–Weinberg equilibrium using the chi-squared test. For categorical variables, the chi-squared test or Fisher exact test was employed, depending on the observed frequency in each case. Continuous variables were compared using Student t-test or the Mann–Whitney test as appropriate. The association between cardiac toxicity and genotype was assessed through logistic regression analysis. Odds ratios (ORs) and P-values were calculated, adjusting for confounding variables such as menopausal status and anthracycline use. Survival curves were generated using the Kaplan–Meier method and compared using the log-rank test. Cox proportional hazards analysis was used to determine whether clinical factors (age, menopausal status, anthracycline use or stage) and/or HER2 655 A > G polymorphisms are significantly associated with outcomes. P-values < 0.05 were considered statistically significant. All analyses were conducted using SPSS version 28 (SPSS Inc., Chicago, Illinois, USA).
Results
Demographic and clinical features
In this study, 88 HER2-positive breast cancer patients undergoing Trastuzumab treatment were included. The average age of the participants was 50 years (± 11.6), with 46.6% being postmenopausal women. Out of these patients, 19 (21.6%) exhibited cardiotoxicity.
Association of the HER2 655 (A > G) polymorphism with cardiac toxicity
Genotype frequencies for HER2/neu 655 (53 AA, 32 AG, and 3 GG) were in Hardy–Weinberg equilibrium (p = 0.488). Due to its small size, the GG genotype group was excluded from further analysis. Table 1 indicates that the clinical characteristics between the AA and AG groups were homogeneous, except in the incidence of cardiotoxicity. Logistic regression, adjusted for menopausal status and anthracycline use, revealed an odds ratio for cardiotoxicity of 4.42 (95% CI 1.46–13.40, p = 0.009) in heterozygote AG versus AA patients (Table 2). Consequently, patients with the AG genotype have a significantly higher risk of experiencing cardiotoxicity.
Association of genotype, LVEF, and cardiac toxicity
Table 3 presents the relationship between the HER2-655 A > G genetic variant, cardiotoxicity, baseline LVEF, and the lowest LVEF recorded during patient follow-up, assessed by echocardiography or isotopic ventriculography. Among the 19 patients who developed cardiotoxicity, 12 had the AG genotype, with a mean baseline LVEF of 60.2. In contrast, of the 66 patients who did not experience cardiotoxicity, 46 were of the AA genotype. No significant differences were observed in the HER2-655 genotype related to LVEF in either the cardiotoxicity or non-cardiotoxicity groups. Moreover, no significant differences were found in mean baseline LVEF when comparing patients who developed cardiotoxicity to those who did not.
Influence of HER2 655 A > G polymorphism on disease-free survival and overall survival
The influence of the HER2 A > G 655 polymorphism on the survival of HER2-positive breast cancer patients was evaluated using Kaplan–Meier analysis, with a mean follow-up duration of 83.1 months. No statistical relationship was observed between disease-free survival (DFS) or overall survival (OS) and the genotype, regardless of whether the data were adjusted for stage (Table 4) or not (Fig. 2). Cox proportional hazards analysis was employed to ascertain whether clinical factors (age, menopausal status, anthracycline use or stage) and/or the HER2 655 A > G polymorphism significantly influenced outcomes (Table 5). It was found that only stage and menopausal status were associated with DFS, while stage and age correlated with OS. Notably, the AA/AG polymorphism did not significantly affect survival outcomes.
Discussion
Trastuzumab treatment has significantly improved outcomes for patients with HER2-positive breast cancer. However, a subset of these patients develops cardiac toxicity [5, 6]. Given the risks of adverse effects of anti-HER2 therapy, predicting which patients might develop cardiac toxicity is crucial for preventing cardiac complications. Our study was conducted in a cohort of Spanish patients of Caucasian descent, and the observed genotype frequencies were consistent with Hardy–Weinberg equilibrium. To the best of our knowledge, no study has indicated significant divergences in allelic or genotypic frequencies associated with the HER2 655 A > G polymorphism within the Spanish population. However, relevant data have been documented in other populations. A study encompassing premenopausal women of diverse ethnic backgrounds, including Ghanaian, Caucasian, African American, Kenyan, Filipino, Saudi Arabian, and Sudanese, revealed that the homozygous G/G genotype exhibited a higher prevalence among Caucasians (5.4%) and African Americans (5.4%) compared to Saudi subjects (2.0%) and Chinese subjects (0.3%). It is noteworthy that this genotype was not detected in any of the three studied continental African populations (Ghanaian, Kenyan, Sudanese). Additionally, no statistically significant differences were evidenced in the frequency of the HER-2 allele between African Americans and Caucasians (P > 0.49) [30] In another study conducted on the Greek population, which included 45 women diagnosed with breast cancer (32 Christians and 13 Muslims) and 56 control cases (43 Christians and 13 Muslims), it was observed that the frequency of the G allele and genotypes containing G was notably higher among Muslims compared to Christians (p = 0.020 and p = 0.008, respectively). Within the Greek Christian population, the risk of breast cancer demonstrated a fivefold increase with the Val/Val genotype and a 3.1-fold increase with the Ile/Val or Val/Val genotypes (95% CI, 1.3–18.4; p = 0.017 and OR, 3.1; 95% CI, 1.2–8.3; p = 0.025; respectively) when compared to the homozygous A/A genotype [31].
Our results indicate that the HER2 655 A > G polymorphism is a genetic predictor of Trastuzumab-induced cardiotoxicity in HER2-positive breast cancer patients. In our study, cardiotoxicity was assessed based on LVEF, which was measured using either echocardiography or isotopic ventriculography. Patients with the HER2-AG genotype are at a higher risk for cardiotoxicity. Other studies examining the impact of this polymorphism in HER2-positive breast cancer patients on cardiotoxicity under Trastuzumab therapy have yielded varying results (Table 6).
In one study exploring the HER2 655 A > G polymorphism, a potential link was investigated between this polymorphism and the occurrence of cardiotoxicity. Cardiotoxicity was assessed through a multiple-gated acquisition scan conducted every 3 months. Among patients treated with Trastuzumab, cardiotoxicity was identified in 8.2% of those with the AG genotype. It was hypothesized that cells expressing the G isoform might experience increased proliferation rates and higher susceptibility to Trastuzumab. Consequently, it was suggested that the presence of the G allele could lead to a dependency of cardiomyocytes on HER2 signaling, resulting in heightened sensitivity to Trastuzumab [27].
A separate investigation involving 78 HER2-positive patients treated with Trastuzumab observed cardiotoxicity in 19.23% of cases. This rate aligns closely with the cardiotoxicity rate observed in our study. Of these, 40% had the AA genotype, while 60% had the AG genotype, a statistically significant difference. The findings concluded that patients with the 655 A > G polymorphism were at an increased risk of developing cardiotoxicity during Trastuzumab treatment [26]. Further analysis focused on the polymorphism in another group of patients found that 71% of patients had the A/A genotype, 25% were A/G, and 4% were G/G. 33% of patients with the A/G genotype exhibited cardiac toxicity, compared to only 8% of patients with the A/A genotype. These differences were statistically significant, supporting the idea that the HER2 A/G genotype could be considered a risk factor for cardiotoxicity during Trastuzumab treatment [32]. In another study conducted in China with 91 patients treated with anthracycline and trastuzumab combined adjuvant chemotherapy, 28.6% developed cardiotoxicity, while 71.4% did not. The patients were assessed at M0, M3, M6, M9, M12, and M15 using an ultrasonic cardiogram, and LVEF was identified through echocardiography. The incidence of cardiotoxicity exhibited a notable increase in patients harboring AG/GG genotypes at the HER2 codon 655, as opposed to individuals with the AA genotype at the same codon (P = 0.007) [33].
Another study, conducted in 132 patients, examined various polymorphisms, including HER2 655 A > G, noted that patients with the G allele in their genotype (A/G + G/G) had a significantly higher number of cardiac alterations compared to those with the A/A genotype. LVEF was assessed through multiple-gated acquisition scanning or echocardiography. Measurements were taken before the administration of trastuzumab and at 1, 6, 9, 12, 16, and 20 months after the initial trastuzumab administration, and subsequently at the 5-year follow-up [34]. A meta-analysis consolidating data from several studies [26, 27, 32, 34] supported the conclusion of a potential link between Trastuzumab-induced cardiotoxicity and the HER2-AG genetic variant. The absence of cardiotoxicity in GG patients was noted to be due to the low representation of this genotype [26]. Similarly, in our study, only 3 patients with the GG genotype were present, which is insufficient for statistical analysis. However, interestingly, two of these patients relapsed, and one died. While the data is limited, this observation could be intriguing for future studies.
However, other studies have not found an association between cardiotoxicity and the genetic variant of codon 655 [35, 36]. In a study with 140 patients treated with Trastuzumab, 29 experienced cardiotoxicity. The AA variant was present in 68% of the patients, AG in 29%, and GG in 3%. No significant relationship was observed between the codon 655 polymorphism and cardiotoxicity (p = 0.96). However, a significant connection was found between cardiotoxicity and codon 1170 when the genotype was Pro/Pro, compared to Pro/Ala or Ala/Ala cases [36].
Our results show a correlation between the HER2 A > G polymorphism and Trastuzumab-induced cardiotoxicity. The model proposed by Beauclair et al. could explain this phenomenon. They found a difference in tumor induction between the G and A alleles and discovered that G-carrying clones exhibited higher sensitivity to Trastuzumab. Experimental results indicate that cells with the G allele have enhanced growth capability and increased sensitivity to Trastuzumab, suggesting that the presence of the G allele could result in cardiomyocytes particularly dependent on HER2 signaling and, therefore, highly sensitive to Trastuzumab [27]. An alternative genetic model linked cardiotoxicity to the HER2 A > G genotype, proposing that patients with both AA and GG alleles have a similar risk of not experiencing cardiotoxicity compared to heterozygous (AG) patients. The HER2 655 A > G polymorphism would cause alterations in the balance between the active and inactive states of the HER2 protein in the body [23].
In our study, we also analyzed the relationship between different HER2 655 genotypes and disease stages (I, II, or III) in the context of DFS and OS. Our findings did not reveal any significant statistical associations, indicating that the A > G polymorphism may not impact patient survival in these cases. To the best of our knowledge, little is known about the potential association between the HER2 655 A > G genotype and DFS in HER2-positive status.
In contrast, another study examining 71 patients with HER2-positive breast cancer treated with Trastuzumab found that individuals with AG or GG genotypes had poorer DFS than those with the AA genotype (P = 0.01) [37]. A study involving HER2-positive patients in an early-stage undergoing adjuvant chemotherapy and/or endocrine therapy without Trastuzumab treatment reported that those with AG or GG genotypes had significantly poorer DFS compared to the AA genotype (P = 0.037). However, among 212 patients treated with chemotherapy and Trastuzumab, the AG or GG genotypes were associated with better DFS than the AA genotype (5-year DFS, P = 0.008), suggesting that the HER2 655 A > G polymorphism influences the HER2 gene function in HER2-positive breast cancers, with the G variant carriers having an aggressive phenotype but showing sensitivity to Trastuzumab. The chemo backbone utilized by 212 patients in this study was adjuvant treatment with trastuzumab combined with adjuvant chemotherapy, which included an anthracycline-based regimen (n = 23), an anthracycline-based regimen followed by taxanes (n = 131), or a taxane-based regimen (n = 58) [38]. In the patients participating in our study, had early-stage breast cancer and all of them were treated with trastuzumab. Additionally, 57 patients were treated with anthracyclines and taxanes, 15 patients were treated with anthracyclines alone and 3 patients were treated with taxanes alone. Similarly to Han et al., we did not find significant differences regarding DFS or OS in patients treated with trastuzumab. The reason for this outcome could be, as indicated by Han et al., because this drug counteracts the negative effect of carrying the G variant. Consistently to our study and Han et al. study, other studies, found no significant differences in DFS were observed between genotypes [27, 39].
Finally, different clinical characteristics with respect to DFS and OS were analyzed. Having a more advanced stage was significant in relation to DFS (P < 0.001) and also for OS (P = 0.007) when comparing stage III with stage II, as found in other studies [38, 40, 41]. This study also found significance for DFS (P = 0.027), indicating that postmenopausal patients have a higher risk of progression, as seen in Goss et al., where premenopausal patients treated with letrozole had a higher DFS (96.5 vs 93.9%) [42]. On the other hand, age, specifically being over 50 years old, was significant for OS (P = 0.035), mirroring other studies where age was a significant factor in OS [43, 44]. However, the HER2 655 A > G polymorphism (AA vs AG) was not associated with survival.
One limitation of our study is the small number of patients, especially in the GG genotype group, where only three patients were included. Notably, among these, two patients experienced a relapse, and one died. While this limited sample size constrains the robustness of our analysis, these observations suggest potential avenues for further research. This study significantly contributes to the ongoing debate about the cardiotoxic potential of trastuzumab in patients with the HER2 655 A > G polymorphism. By offering valuable data, our research clarifies the relationship between this specific polymorphism and the adverse effects of trastuzumab. The novelty of our work lies in its comprehensive analysis of the influence of the 655 A > G polymorphism on trastuzumab response and its integration with survival data in relation to clinical characteristics. This approach enhances our understanding of the polymorphism impact, underscoring the need for personalized treatment strategies in HER2-positive breast cancer.
Conclusion
Our study supports the role of the HER2 655 A > G polymorphism as a potential genetic risk factor for Trastuzumab-induced cardiotoxicity in patients with HER2-positive breast cancer. However, this polymorphism was not associated with survival. Further research, incorporating a larger patient cohort that could lead to an increase in the number of GG genotype patients, is required to validate the genetic model. This could lead to the establishment of a robust pharmacogenetic marker that could assist physicians in managing Trastuzumab-induced cardiotoxicity more effectively.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
Abbreviations
- DFS:
-
Disease-free survival
- HER2:
-
Human epidermal growth factor receptor 2
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- OS:
-
Overall survival
References
Sirkisoon SR, Carpenter RL, Rimkus T, Miller L, Metheny-Barlow L, Lo H-W. EGFR and HER2 signaling in breast cancer brain metastasis. Front Biosci (Elite Ed). 2016;8:245–63. https://doi.org/10.2741/E765.
Chen W, Li F-X, Lu D-L, Jiang J, Li J. Differences between the efficacy of HER2(2+)/FISH-positive and HER2(3+) in breast cancer during dual-target neoadjuvant therapy. Breast. 2023;71:69–73. https://doi.org/10.1016/j.breast.2023.07.008.
Chang C-H, Jung C-J, Huang Y-M, Chiao L, Chang Y-L, Hsieh S-C, et al. The first reported case of trastuzumab induced interstitial lung disease associated with anti-neutrophil cytoplasmic antibody vasculitis - a case report and a prospective cohort study on the usefulness of neutrophil derived biomarkers in monitoring vasculitis disease activity during follow-up. Breast. 2022;61:35–42. https://doi.org/10.1016/j.breast.2021.11.016.
Hudis CA. Trastuzumab — mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. https://doi.org/10.1056/NEJMra043186.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83. https://doi.org/10.1056/NEJMoa0910383.
Costa RB, Kurra G, Greenberg L, Geyer CE. Efficacy and cardiac safety of adjuvant trastuzumab-based chemotherapy regimens for HER2-positive early breast cancer. Ann Oncol. 2010;21:2153–60. https://doi.org/10.1093/annonc/mdq096.
Jones SE, Collea R, Paul D, Sedlacek S, Favret AM, Gore I, et al. Adjuvant docetaxel and cyclophosphamide plus trastuzumab in patients with HER2-amplified early stage breast cancer: a single-group, open-label, phase 2 study. Lancet Oncol. 2013;14:1121–8. https://doi.org/10.1016/S1470-2045(13)70384-X.
Di Cosimo S. Heart to heart with trastuzumab: a review on cardiac toxicity. Target Oncol. 2011;6:189–95. https://doi.org/10.1007/s11523-011-0203-8.
Schmitz KR, Ferguson KM. Interaction of antibodies with ErbB receptor extracellular regions. Exp Cell Res. 2009;315:659–70. https://doi.org/10.1016/j.yexcr.2008.10.008.
Vuger AT, Tiscoski K, Apolinario T, Cardoso F. Anthracyclines in the treatment of early breast cancer friend or foe? Breast. 2022;65:67–76. https://doi.org/10.1016/j.breast.2022.06.007.
Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21. https://doi.org/10.1200/JCO.2002.20.5.1215.
Pondé NF, Lambertini M, de Azambuja E. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open. 2016;1: e000073. https://doi.org/10.1136/esmoopen-2016-000073.
Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859–65. https://doi.org/10.1200/JCO.2006.09.1611.
Abdel-Qadir H, Bobrowski D, Zhou L, Austin PC, Calvillo-Argüelles O, Amir E, et al. Statin exposure and risk of heart failure after anthracycline- or trastuzumab-based chemotherapy for early breast cancer: a propensity score-matched cohort study. J Am Heart Assoc. 2021;10: e018393. https://doi.org/10.1161/JAHA.119.018393.
Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol. 2006;41:228–35. https://doi.org/10.1016/j.yjmcc.2006.04.007.
Zeglinski M, Ludke A, Jassal DS, Singal PK. Trastuzumab-induced cardiac dysfunction: a ‘dual-hit.’ Exp Clin Cardiol. 2011;16:70–4.
Blancas I, Martín-Pérez FJ, Garrido JM, Rodríguez-Serrano F. NT-proBNP as predictor factor of cardiotoxicity during trastuzumab treatment in breast cancer patients. Breast. 2020;54:106–13. https://doi.org/10.1016/j.breast.2020.09.001.
Çetin S, Babaoğlu K, Başar EZ, Deveci M, Çorapçıoğlu F. Subclinical anthracycline-induced cardiotoxicity in long-term follow-up of asymptomatic childhood cancer survivors: Assessment by speckle tracking echocardiography. Echocardiography. 2018;35:234–40. https://doi.org/10.1111/echo.13743.
Eidem BW. Identification of anthracycline cardiotoxicity: left ventricular ejection fraction is not enough. J Am Soc Echocardiogr. 2008;21:1290–2. https://doi.org/10.1016/j.echo.2008.10.008.
Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: is our ear really to the ground? J Clin Oncol. 2008;26:1201–3. https://doi.org/10.1200/JCO.2007.14.8742.
Jacobs LD, Salgo IS, Goonewardena S, Weinert L, Coon P, Bardo D, et al. Rapid online quantification of left ventricular volume from real-time three-dimensional echocardiographic data. Eur Heart J. 2006;27:460–8. https://doi.org/10.1093/eurheartj/ehi666.
Altena R, Bajalica-Lagercrantz S, Papakonstantinou A. Pharmacogenomics for prediction of cardiovascular toxicity: landscape of emerging data in breast cancer therapies. Cancers (Basel). 2022;14:4665. https://doi.org/10.3390/cancers14194665.
Fleishman SJ, Schlessinger J, Ben-Tal N. A putative molecular-activation switch in the transmembrane domain of erbB2. Proc Natl Acad Sci U S A. 2002;99:15937–40. https://doi.org/10.1073/pnas.252640799.
Papewalis J, Nikitin AYu, Rajewsky MF. G to A polymorphism at amino acid codon 655 of the human erbB-2/HER2 gene. Nucleic Acids Res. 1991;19:5452.
Wang H, Liu L, Lang Z, Guo S, Gong H, Guan H, et al. Polymorphisms of ERBB2 and breast cancer risk: a meta-analysis of 26 studies involving 35,088 subjects. J Sur Oncol. 2013;108:337–41. https://doi.org/10.1002/jso.23386.
Gómez Peña C, Dávila-Fajardo CL, Martínez-González LJ, Carmona-Sáez P, Soto Pino MJ, Sánchez Ramos J, et al. Influence of the HER2 Ile655Val polymorphism on trastuzumab-induced cardiotoxicity in HER2-positive breast cancer patients: a meta-analysis. Pharmacogenet Genom. 2015;25:388–93. https://doi.org/10.1097/FPC.0000000000000149.
Beauclair S, Formento P, Fischel JL, Lescaut W, Largillier R, Chamorey E, et al. Role of the HER2 [Ile655Val] genetic polymorphism in tumorogenesis and in the risk of trastuzumab-related cardiotoxicity. Ann Oncol. 2007;18:1335–41. https://doi.org/10.1093/annonc/mdm181.
Alexandraki A, Papageorgiou E, Zacharia M, Keramida K, Papakonstantinou A, Cipolla CM, et al. New Insights in the era of clinical biomarkers as potential predictors of systemic therapy-induced cardiotoxicity in women with breast cancer: a systematic review. Cancers (Basel). 2023;15:3290. https://doi.org/10.3390/cancers15133290.
Nicol M, Baudet M, Cohen-Solal A. Subclinical left ventricular dysfunction during chemotherapy. Card Fail Rev. 2019;5:31–6. https://doi.org/10.15420/cfr.2018.25.1.
Ameyaw M-M, Tayeb M, Thornton N, Folayan G, Tariq M, Mobarek A, et al. Ethnic variation in the HER-2 codon 655 genetic polymorphism previously associated with breast cancer. J Hum Genet. 2002;47:172–5. https://doi.org/10.1007/s100380200019.
Papadopoulou E, Simopoulos K, Tripsianis G, Tentes I, Anagnostopoulos K, Sivridis E, et al. Allelic imbalance of HER-2 codon 655 polymorphism among different religious/ethnic populations of northern Greece and its association with the development and the malignant phenotype of breast cancer. Neoplasma. 2007;54:365–73.
Lemieux J, Diorio C, Côté M-A, Provencher L, Barabé F, Jacob S, et al. Alcohol and HER2 polymorphisms as risk factor for cardiotoxicity in breast cancer treated with trastuzumab. Anticancer Res. 2013;33:2569–76.
Tan L, Su X, Li X, Li H, Hu B. Correlation of HER2 codon 655 polymorphism with cardiotoxicity risk in Chinese HER2-positive breast cancer patients undergoing epirubicin/cyclophosphamide followed by docetaxel plus trastuzumab adjuvant chemotherapy. Int J Clin Exp Pathol. 2020;13:286–94.
Roca L, Diéras V, Roché H, Lappartient E, Kerbrat P, Cany L, et al. Correlation of HER2, FCGR2A, and FCGR3A gene polymorphisms with trastuzumab related cardiac toxicity and efficacy in a subgroup of patients from UNICANCER-PACS 04 trial. Breast Cancer Res Treat. 2013;139:789–800. https://doi.org/10.1007/s10549-013-2587-x.
Serie DJ, Crook JE, Necela BM, Dockter TJ, Wang X, Asmann YW, et al. Genome-wide association study of cardiotoxicity in the NCCTG N9831 (Alliance) adjuvant trastuzumab trial. Pharmacogenet Genom. 2017;27:378. https://doi.org/10.1097/FPC.0000000000000302.
Stanton SE, Ward MM, Christos P, Sanford R, Lam C, Cobham MV, et al. Pro1170 Ala polymorphism in HER2-neu is associated with risk of trastuzumab cardiotoxicity. BMC Cancer. 2015;15:267. https://doi.org/10.1186/s12885-015-1298-6.
Furrer D, Jacob S, Michaud A, Provencher L, Lemieux J, Diorio C. Association of tobacco use, alcohol consumption and HER2 polymorphisms with response to trastuzumab in HER2-positive breast cancer patients. Clin Breast Cancer. 2018;18:e687–94. https://doi.org/10.1016/j.clbc.2017.11.012.
Han X, Diao L, Xu Y, Xue W, Ouyang T, Li J, et al. Association between the HER2 Ile655Val polymorphism and response to trastuzumab in women with operable primary breast cancer. Ann Oncol. 2014;25:1158–64. https://doi.org/10.1093/annonc/mdu111.
Peddi PF, Fasching PA, Liu D, Quinaux E, Robert NJ, Valero V, et al. Genetic polymorphisms and correlation with treatment-induced cardiotoxicity and prognosis in patients with breast cancer. Clin Cancer Res. 2022;28:1854–62. https://doi.org/10.1158/1078-0432.CCR-21-1762.
Saadatmand S, Bretveld R, Siesling S, Tilanus-Linthorst MMA. Influence of tumour stage at breast cancer detection on survival in modern times: population based study in 173 797 patients. BMJ. 2015;351: h4901. https://doi.org/10.1136/bmj.h4901.
Sant M, Allemani C, Capocaccia R, Hakulinen T, Aareleid T, Coebergh JW, et al. Stage at diagnosis is a key explanation of differences in breast cancer survival across Europe. Int J Cancer. 2003;106:416–22. https://doi.org/10.1002/ijc.11226.
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Livingston RB, et al. Impact of premenopausal status at breast cancer diagnosis in women entered on the placebo-controlled NCIC CTG MA17 trial of extended adjuvant letrozole. Ann Oncol. 2013;24:355–61. https://doi.org/10.1093/annonc/mds330.
Aaltomaa S, Lipponen P, Eskelinen M. Demographic prognostic factors in breast cancer. Acta Oncol. 1992;31:635–40. https://doi.org/10.3109/02841869209083845.
Abdel-Razeq H, Iweir S, Abdel-Razeq R, Rahman FA, Almasri H, Bater R, et al. Differences in clinicopathological characteristics, treatment, and survival outcomes between older and younger breast cancer patients. Sci Rep. 2021;11:14340. https://doi.org/10.1038/s41598-021-93676-w.
Acknowledgements
The content of this article is part of the PhD thesis of Marina Linares-Rodríguez within the Doctoral Program in Biomedicine of the University of Granada, Spain.
Funding
Funding for open access publishing: Universidad de Granada/CBUA. This work was supported by a grant from the Consejería de Salud y Familias-Junta de Andalucía (PECART-0207–2020).
Author information
Authors and Affiliations
Contributions
The conception and overall direction of this study were led by IB and FR-S. Data collection and analysis were collaboratively undertaken by all authors. The initial draft of the manuscript was composed by ML-R and CM-B, and all authors provided feedback on previous versions. Each author has reviewed and given their approval to the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Isabel Blancas received grants and support by Roche.
Ethical approval, Human participants and/or animals and Informed consent
Informed, written consent was obtained from all participants involved in the study. Additionally, the research received approval from the Provincial Ethical Committee of Granada, ensuring adherence to ethical standards in the conduct of this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blancas, I., Linares-Rodríguez, M., Martín-Bravo, C. et al. HER2/neu 655 polymorphism, trastuzumab-induced cardiotoxicity, and survival in HER2-positive breast cancer patients. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03512-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03512-6