Abstract
Prostate cancer (PCa) is the second most prevalent cancer in men. In 2020, approximately 1,414,259 new cases were reported that accounted for 3,75,324 deaths (Sung et al. in CA 71:209–249, 2021). PCa is often asymptomatic at early stages; hence, routine screening and monitoring based on reliable biomarkers is crucial for early detection and assessment of cancer progression. Early diagnosis of disease is key step in reducing PCa-induced mortality. Biomarkers such as PSA have played vital role in reducing recent PCa deaths. Recent research has identified many other biomarkers and also refined PSA-based tests for non-invasive diagnosis of PCa in patients. Despite progress in screening methods, an important issue that influences treatment is heterogeneity of the cancer in different individuals, necessitating personalized treatment. Currently, focus is to identify biomarkers that can accurately diagnose PCa at early stage, indicate the stage of the disease, metastatic nature and chances of survival based on individual patient profile (Fig. 1).
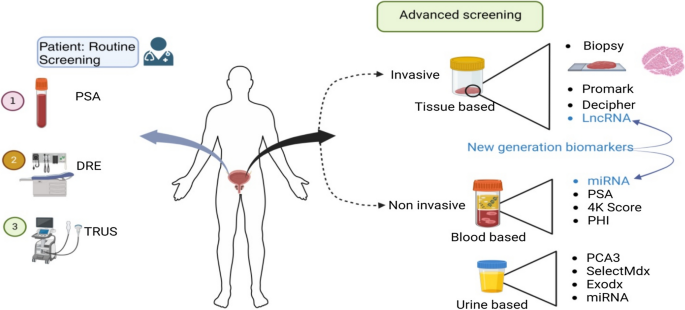
Graphical abstract




Similar content being viewed by others
Data availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71(3):209–49.
Rice SM, Oliffe JL, Kelly MT, Cormie P, Chambers S, Ogrodniczuk JS, et al. Depression and prostate cancer: examining comorbidity and male-specific symptoms. Am J Mens Health. 2018;12(6):1864–72.
Madu CO, Lu Y. Novel diagnostic biomarkers for prostate cancer. J Cancer. 2010;1:150.
Gutman AB, Gutman EB. An “acid” phosphatase occurring in the serum of patients with metastasizing carcinoma of the prostate gland. J Clin Investig. 1938;17(4):473–8.
Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol. 1979;17(2):159–63.
Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317(15):909–16.
Mettlin C, Lee F, Drago J, Murphy GP. The American cancer society national prostate cancer detection project. Findings on the detection of early prostate cancer in 2425 men. Cancer. 1991;67(12):2949–58.
Basler JW, Thompson IM. Lest we abandon digital rectal examination as a screening test for prostate cancer. J Natl Cancer Inst. 1998;90(23):1761–3.
Mahon SM. Screening for prostate cancer: informing men about their options. Clin J Oncol Nurs. 2005;9(5):625.
Hara M. Some physiochemical characteristics of gamma-semino-protein: An antigenic component specific for human plasma. Jpn J Legal Med. 1971;25:322–4.
Rao AR, Motiwala HG, Karim OMA. The discovery of prostate-specific antigen. BJU Int. 2008;101(1):5–10.
Klotz L. Active surveillance for prostate cancer: overview and update. Curr Treat Options Oncol. 2013;14:97–108.
Panzone J, Byler T, Bratslavsky G, Goldberg H. Applications of focused ultrasound in the treatment of genitourinary cancers. Cancers. 2022;14(6):1536.
Crawford ED, Scholz MC, Kar AJ, Fegan JE, Haregewoin A, Kaldate RR, et al. Cell cycle progression score and treatment decisions in prostate cancer: results from an ongoing registry. Curr Med Res Opin. 2014;30(6):1025–31.
Welti J, Rodrigues DN, Sharp A, Sun S, Lorente D, Riisnaes R, et al. Analytical validation and clinical qualification of a new immunohistochemical assay for androgen receptor splice variant-7 protein expression in metastatic castration-resistant prostate cancer. Eur Urol. 2016;70(4):599–608.
Van der Kwast TH. Prognostic prostate tissue biomarkers of potential clinical use. Virchows Arch. 2014;464(3):293–300.
Bazzichetto C, Conciatori F, Pallocca M, Falcone I, Fanciulli M, et al. PTEN as a prognostic/predictive biomarker in cancer: an unfulfilled promise? Cancers. 2019;11(4):435.
Coradduzza D, Solinas T, Balzano F, Culeddu N, Rossi N, Cruciani S, et al. miRNAs as molecular biomarkers for prostate cancer. J Mol Diagn. 2022;24(11):1171–80.
Rana S, Valbuena GN, Curry E, Bevan CL, Keun HC. MicroRNAs as biomarkers for prostate cancer prognosis: a systematic review and a systematic reanalysis of public data. Br J Cancer. 2022;126(3):502–13.
Wang W, Wang M, Wang L, Adams TS, Tian Y, Xu J. Diagnostic ability of% p2PSA and prostate health index for aggressive prostate cancer: a meta-analysis. Sci Rep. 2014;4(1):5012.
De La Calle C, Patil D, Wei JT, Scherr DS, Sokoll L, Chan DW, et al. Multicenter evaluation of the prostate health index to detect aggressive prostate cancer in biopsy naive men. J Urol. 2015;194(1):65–72.
Stephan C, Ralla B, Jung K. Prostate-specific antigen and other serum and urine markers in prostate cancer. Biochem Biophys Acta. 2014;1846(1):99–112.
Filella X, Gimenez N. Evaluation of [− 2] proPSA and Prostate Health Index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med. 2013;51(4):729–39.
Hernández J, Thompson IM. Prostate-specific antigen: a review of the validation of the most commonly used cancer biomarker. Cancer. 2004;101(5):894–904.
Punnen S, Freedland SJ, Polascik TJ, Loeb S, Risk MC, Savage S, et al. A multi-institutional prospective trial confirms noninvasive blood test maintains predictive value in African American men. J Urol. 2018;199(6):1459–63.
Hayes VM, Bornman MR. Prostate cancer in Southern Africa: does Africa hold untapped potential to add value to the current understanding of a common disease? J Global Oncol. 2018;4:1–7.
Voigt JD, Dong Y, Linder V, Zappala S. Use of the 4Kscore test to predict the risk of aggressive prostate cancer prior to prostate biopsy: Overall cost savings and improved quality of care to the us healthcare system. Rev Urol. 2017;19(1):1.
Zappala SM, Scardino PT, Okrongly D, Linder V, Dong Y. Clinical performance of the 4Kscore Test to predict high-grade prostate cancer at biopsy: A meta-analysis of us and European clinical validation study results. Rev urol. 2017;19(3):149.
Lin DW, Newcomb LF, Brown MD, Sjoberg DD, Dong Y, Brooks JD, et al. Evaluating the four kallikrein panel of the 4Kscore for prediction of high-grade prostate cancer in men in the Canary Prostate Active Surveillance Study. Eur Urol. 2017;72(3):448–54.
Stattin P, Vickers AJ, Sjoberg DD, Johansson R, Granfors T, Johansson M, et al. Improving the specificity of screening for lethal prostate cancer using prostate-specific antigen and a panel of kallikrein markers: a nested case–control study. Eur Urol. 2015;68(2):207–13.
Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH, et al. A multicenter study of [-2] pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol. 2011;185(5):1650–5.
Porzycki P, Ciszkowicz E. Modern biomarkers in prostate cancer diagnosis. Central Eur J Urol. 2020;73(3):300.
Filella X, Foj L, Augé JM, Molina R, Alcover J. Clinical utility of% p2PSA and prostate health index in the detection of prostate cancer. Clin Chem Lab Med. 2014;52(9):1347–55.
Loeb S, Sanda MG, Broyles DL, Shin SS, Bangma CH, Wei JT, et al. The prostate health index selectively identifies clinically significant prostate cancer. J Urol. 2015;193(4):1163–9.
Hussein AA, Baban R, Hussein A. Prostate-specific antigen and free prostate-specific antigen/prostate-specific antigen ratio in patients with benign prostatic hyperplasia and prostate cancer. Baghdad J Biochem Appl Biol Sci. 2020;1(01):18–26.
Eyrich NW, Morgan TM, Tosoian JJ. Biomarkers for detection of clinically significant prostate cancer: contemporary clinical data and future directions. Transl Androl Urol. 2021;10(7):3091.
Cui Y, Cao W, Li Q, Shen H, Liu C, Deng J, et al. Evaluation of prostate cancer antigen 3 for detecting prostate cancer: a systematic review and meta-analysis. Sci Rep. 2016;6(1):25776.
Kornberg Z, Cooperberg MR, Spratt DE, Feng FY. Genomic biomarkers in prostate cancer. Transl Androl Urol. 2018;7(3):459.
Tutrone R, Donovan MJ, Torkler P, Tadigotla V, McLain T, Noerholm M, et al. Clinical utility of the exosome based ExoDx Prostate (IntelliScore) EPI test in men presenting for initial Biopsy with a PSA 2–10 ng/mL. Prostate Cancer Prostatic Dis. 2020;23(4):607–14.
Marrugo-Ramírez J, Mir M, Samitier J. Blood-based cancer biomarkers in liquid biopsy: a promising non-invasive alternative to tissue biopsy. Int J Mol Sci. 2018;19(10):2877.
Jamaspishvili T, Kral M, Khomeriki I, Student V, Kolar Z, Bouchal J. Urine markers in monitoring for prostate cancer. Prostate Cancer Prostatic Dis. 2010;13(1):12–9.
Tomlins SA, Bjartell A, Chinnaiyan AM, Jenster G, Nam RK, Rubin MA, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol. 2009;56(2):275–86.
Hessels D, Schalken JA. The use of PCA3 in the diagnosis of prostate cancer. Nat Rev Urol. 2009;6(5):255–61.
Nicholson A, Mahon J, Boland A, Beale S, Dwan K, Fleeman N, et al. The clinical effectiveness and cost-effectiveness of the PROGENSA® prostate cancer antigen 3 assay and the Prostate Health Index in the diagnosis of prostate cancer: a systematic review and economic evaluation. Health Technol Assess. 2015;19(87):1–31.
Munroz Rodríguez SVM, García-Perdomo HA. Diagnostic accuracy of prostate cancer antigen 3 (PCA3) prior to first prostate biopsy: A systematic review and meta-analysis. Can Urol Assoc J. 2020;14(5):E214.
Kristiansen G. Diagnostic and prognostic molecular biomarkers for prostate cancer. Histopathology. 2012;60(1):125–41.
Kretschmer A, Tilki D. Biomarkers in prostate cancer–current clinical utility and future perspectives. Crit Rev Oncol Hematol. 2017;120:180–93.
Alford AV, Brito JM, Yadav KK, Yadav SS, Tewari AK, Renzulli J. The use of biomarkers in prostate cancer screening and treatment. Rev Urol. 2017;19(4):221.
Kohaar I, Petrovics G, Srivastava S. A rich array of prostate cancer molecular biomarkers: opportunities and challenges. Int J Mol Sci. 2019;20(8):1813.
Fujita K, Nonomura N. Urinary biomarkers of prostate cancer. Int J Urol. 2018;25(9):770–9.
Demichelis F, Fall K, Perner S, Andrén O, Schmidt F, Setlur SR, et al. TMPRSS2: ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26(31):4596–9.
McKiernan J, Donovan MJ, O’Neill V, Bentink S, Noerholm M, Belzer S, et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2016;2(7):882–9.
Humphrey PA. Histopathology of prostate cancer. Cold Spring Harb Perspect Med. 2017;7(10):a030411.
Basourakos SP, Tzeng M, Lewicki PJ, Patel K, Awamlh BAHA, Venkat S, et al. Tissue-based biomarkers for the risk stratification of men with clinically localized prostate cancer. Front Oncol. 2021;11:676716.
Lokeshwar SD, Klaassen Z, Saad F. Treatment and trials in non-metastatic castration-resistant prostate cancer. Nat Rev Urol. 2021;18(7):433–42.
Moschini M, Spahn M, Mattei A, Cheville J, Karnes RJ. Incorporation of tissue-based genomic biomarkers into localized prostate cancer clinics. BMC Med. 2016;14(1):1–7.
Khoo A, Liu LY, Nyalwidhe JO, Semmes OJ, Vesprini D, Downes MR, et al. Proteomic discovery of non-invasive biomarkers of localized prostate cancer using mass spectrometry. Nat Rev Urol. 2021;18(12):707–24.
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–29.
Ahmed HU, Bosaily AES, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Erho N, Crisan A, Vergara IA, Mitra AP, Ghadessi M, Buerki C, et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE. 2013;8(6):e66855.
Spratt DE, Dai DL, Den RB, Troncoso P, Yousefi K, Ross AE, et al. Performance of a prostate cancer genomic classifier in predicting metastasis in men with prostate-specific antigen persistence postprostatectomy. Eur Urol. 2018;74(1):107–14.
Behm-Ansmant I, Rehwinkel J, Izaurralde E. MiRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Quant Biol. 2006;71:523–30.
Pedroza-Torres A, Romero-Córdoba SL, Justo-Garrido M, Salido-Guadarrama I, Rodríguez-Bautista R, Montaño S, et al. MicroRNAs in tumor cell metabolism: roles and therapeutic opportunities. Front Oncol. 2019;9:1404.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci. 2008;105(30):10513–8.
Meiri E, Mueller WC, Rosenwald S, Zepeniuk M, Klinke E, Edmonston TB, et al. A second-generation microRNA-based assay for diagnosing tumor tissue origin. Oncologist. 2012;17(6):801–12.
Li D, Hao X, Song Y. Identification of the key MicroRNAs and the miRNA-mRNA regulatory pathways in prostate cancer by bioinformatics methods. Biomed Res Int. 2018. https://doi.org/10.1155/2018/6204128.
Guo H, Qi RQ, Sheng J, Liu C, Ma H, Wang HX, et al. MiR-155, a potential serum marker of extramammary Paget’s disease. BMC Cancer. 2018;18:1–8.
Wani S, Kaul D, Mavuduru RS, Kakkar N, Bhatia A. Urinary-exosomal miR-2909: a novel pathognomonic trait of prostate cancer severity. J Biotechnol. 2017;259:135–9.
Matin F, Jeet V, Moya L, Selth LA, Chambers S, Yeadon APCB, et al. A plasma biomarker panel of four microRNAs for the diagnosis of prostate cancer. Sci Rep. 2018;8(1):6653.
Leite KR, Morais DR, Reis ST, Viana N, Moura C, Florez MG, et al. MicroRNA 100: a context dependent miRNA in prostate cancer. Clinics. 2013;68:797–802.
Ghamlouche F, Yehya A, Zeid Y, Fakhereddine H, Fawaz J, Liu YN, Abou-Kheir W. MicroRNAs as clinical tools for diagnosis, prognosis, and therapy in prostate cancer. Transl Oncol. 2023;28:101613.
Barceló M, Castells M, Bassas L, Vigués F, Larriba S. Semen miRNAs contained in exosomes as non-invasive biomarkers for prostate cancer diagnosis. Sci Rep. 2019;9(1):13772.
Cuzick J, Swanson GP, Fisher G, Brothman AR, Berney DM, Reid JE, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12(3):245–55.
Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67(1):33–41.
Penney KL, Sinnott JA, Fall K, Pawitan Y, Hoshida Y, Kraft P, et al. mRNA expression signature of Gleason grade predicts lethal prostate cancer. J Clin Oncol. 2011;29(17):2391.
Prensner JR, Iyer MK, Sahu A, Asangani IA, Cao Q, Patel L, et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet. 2013;45(11):1392–8.
Xiong T, Li J, Chen F, Zhang F. PCAT-1: a novel oncogenic long non-coding RNA in human cancers. Int J Biol Sci. 2019;15(4):847.
Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199–208.
Camacho CV, Choudhari R, Gadad SS. Long noncoding RNAs and cancer, an overview. Steroids. 2018;133:93–5.
Qiu M, Xu Y, Wang J, Zhang E, Sun M, Zheng Y, et al. A novel lncRNA, LUADT1, promotes lung adenocarcinoma proliferation via the epigenetic suppression of p27. Cell Death Dis. 2015;6(8):e1858–e1858.
Lemos AEG, Ferreira LB, Batoreu NM, de Freitas PP, Bonamino MH, Gimba ERP. PCA3 long noncoding RNA modulates the expression of key cancer-related genes in LNCaP prostate cancer cells. Tumor Biol. 2016;37:11339–48.
Sanda MG, Feng Z, Howard DH, Tomlins SA, Sokoll LJ, Chan D, et al. Association between combined TMPRSS2:ERG and PCA3 RNA urinary testing and detection of aggressive prostate cancer. JAMA Oncol. 2017;3(8):1085–93. https://doi.org/10.1001/jamaoncol.2017.0177.
Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1(5):391–407.
Prensner JR, Sahu A, Iyer MK, Malik R, Chandler B, Asangani IA, et al. The lncRNAs PCGEM1 and PRNCR1 are not implicated in castration resistant prostate cancer. Oncotarget. 2014;5(6):1434.
Mehra R, Shi Y, Udager AM, Prensner JR, Sahu A, Iyer MK, et al. A novel RNA in situ hybridization assay for the long noncoding RNA SChLAP1 predicts poor clinical outcome after radical prostatectomy in clinically localized prostate cancer. Neoplasia. 2014;16(12):1121–7.
Cozar JM, Robles-Fernandez I, Rodriguez-Martinez A, Puche-Sanz I, Vazquez-Alonso F, Lorente JA, et al. The role of miRNAs as biomarkers in PCa. Mutat Res Rev Mutat Res. 2019;781:165–74.
Allemailem KS, Almatroudi A, Alrumaihi F, Makki Almansour N, Aldakheel FM, Rather RA, et al. Single nucleotide polymorphisms (SNPs) in prostate cancer: its implications in diagnostics and therapeutics. Am J Transl Res. 2021;13(4):3868–89.
Chang HH, Lee CH, Chen YT, Huang CY, Yu CC, Lin VC, et al. Genetic analysis reveals the prognostic significance of the DNA mismatch repair gene MSH2 in advanced prostate cancer. Cancers. 2022;14(1):223.
Lee CH, Pao JB, Lu TL, Lee HZ, Lee YC, Liu CC, et al. Prognostic value of prostaglandin-endoperoxide synthase 2 polymorphisms in prostate cancer recurrence after radical prostatectomy. Int J Med Sci. 2016;13(9):696–700. https://doi.org/10.7150/ijms.16259.
Mangolini A, Rocca C, Bassi C, Ippolito C, Negrini M, Dell’Atti L, et al. Detection of disease-causing mutations in prostate cancer by NGS sequencing. Cell Biol Int. 2022;46(7):1047–61.
Goel S, Bhatia V, Kundu S, Biswas T, Carskadon S, Gupta N, et al. Transcriptional network involving ERG and AR orchestrates Distal-less homeobox-1 mediated prostate cancer progression. Nat Commun. 2021;12(1):5325.
Singh JP, Dagar M, Dagar G, Kumar S, Rawal S, Bagchi G, et al. Activation of GPR56, a novel adhesion GPCR, is necessary for nuclear androgen receptor signaling in prostate cells. PLoS ONE. 2020;15(9):e0226056.
Sánchez Iglesias Á, Morillo Macías V, Picó Peris A, Fuster-Matanzo A, Nogué Infante A, Muelas Soria R, et al. Prostate region-wise imaging biomarker profiles for risk stratification and biochemical recurrence prediction. Cancers. 2023;15(16):4163.
Padhani AR, Schoots IG. Prostate cancer screening—stepping forward with MRI. Eur Radiol. 2023;33(10):6670–6.
Eklund M, Jäderling F, Discacciati A, Bergman M, Annerstedt M, Aly M, et al. MRI-targeted or standard biopsy in prostate cancer screening. N Engl J Med. 2021;385(10):908–20.
Nordström T, Discacciati A, Bergman M, Clements M, Aly M, Annerstedt M, et al. Prostate cancer screening using a combination of risk-prediction, MRI, and targeted prostate biopsies (STHLM3-MRI): a prospective, population-based, randomised, open-label, non-inferiority trial. Lancet Oncol. 2021;22(9):1240–9.
Yaman Agaoglu F, Kovancilar M, Dizdar Y, Darendeliler E, Holdenrieder S, Dalay N, et al. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumor Biol. 2011;32:583–8.
Acknowledgements
We are thankful to Science & Engineering Research Board (SERB) grant CRG/2019/002583.
Funding
Science and Engineering Research Board, CRG/2019/002583, Gargi Bagchi.
Author information
Authors and Affiliations
Contributions
Gargi Bagchi: conceptualization, supervision, review and editing. Versha Dahiya: manuscript writing, preparing figures and tables. Sanjana Hans and Ruchi Kumari: manuscript writing.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical statement
“No ethical approval was required as this study did not involve human participants or laboratory animals.”
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dahiya, V., Hans, S., Kumari, R. et al. Prostate cancer biomarkers: from early diagnosis to precision treatment. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03508-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03508-2




