Abstract
Purpose
HER2-targeted therapies have dramatically improved outcomes of patients with HER2-positive breast cancer (BC), as demonstrated in neoadjuvant trials. This study aims to provide real-world evidence on the use and effectiveness of combined pertuzumab, trastuzumab and chemotherapy (CT) in early-stage HER2-positive BC.
Methods
A retrospective, multicentre study was conducted on patients diagnosed with HER2-positive early BC treated with neoadjuvant pertuzumab and trastuzumab plus CT at 13 Spanish sites. The primary endpoint was pathological complete response (pCR).
Results
A total of 310 patients were included. Pertuzumab and trastuzumab were combined with anthracyclines and taxanes, carboplatin and docetaxel, and taxane-based CT in 77.1%, 16.5%, and 6.5% of patients, respectively. Overall, the pCR rate was 62.2%. The pCR was higher amongst patients with hormone receptor-negative tumours and with tumours expressing higher levels of Ki-67 (> 20%). After postoperative adjuvant treatment, 13.9% of patients relapsed. Those patients who did not achieve pCR, with tumours at advanced stages (III), and with node-positive disease were more likely to experience distant relapse. Median overall survival (OS) and distant disease-free survival (D-DFS) were not reached at the study end. The estimated mean OS and D-DFS times were 7.5 (95% CI 7.3–7.7) and 7.3 (95% CI 7.1–7.5) years, respectively (both were significantly longer amongst patients who achieved pCR). Grade 3–4 anti-HER2 related toxicities were reported in six (1.9%) patients.
Conclusion
Neoadjuvant pertuzumab and trastuzumab plus CT achieve high pCR rates in real-life patients with HER2-positive early BC, showing an acceptable safety profile. Innovative adjuvant strategies are essential in patients at high risk of distant disease recurrence.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer in the world, affecting more than 2 million people and causing nearly 680,000 deaths each year [1]. Amplification of HER2/neu gene or otherwise overexpression of the human epidermal growth factor receptor 2 (HER2) is found in approximately 15–20% of breast cancers [2] and has been classically associated with an aggressive clinical course and poor outcomes [3]. However, the development of HER2-targeted therapies in recent years has substantially improved outcomes in these patients.
The benefits of neoadjuvant BC treatment with chemotherapy (CT), endocrine therapy and/or targeted therapy are well established, as it improves tumour resectability, often reducing the extent of breast and axillary surgery [4]. In addition, neoadjuvant systemic therapy allows an approach to personalised adjuvant treatment based on the pathological response. Pathological complete response (pCR) is defined as the absence of residual invasive cancer of the complete resected breast tumour and all regional lymph nodes (ypT0/Tis ypN0), after completion of neoadjuvant systemic therapy [5]. Achievement of pCR at surgery is correlated with favourable outcomes and is considered a reliable surrogate endpoint for enhanced survival in HER2-positive BC [6].
Trastuzumab represents the cornerstone for neoadjuvant treatment in HER2-positive BC due to its success in several clinical trials. The addition of trastuzumab to conventional neoadjuvant CT showed remarkable improvements in pCR and event-free survival [7, 8]. Nevertheless, many patients are likely to develop resistance to trastuzumab [9]. Pertuzumab has a complementary mechanism of action to that of trastuzumab, which consists of binding the extracellular domain II of HER2, preventing HER2-HER3 dimerization. The NeoSphere trial showed increased pCR rates in patients treated with pertuzumab plus trastuzumab combined with CT compared to those who received only trastuzumab and CT in the neoadjuvant setting [10]. The combination of pertuzumab with trastuzumab and different CT regimens has been investigated in neoadjuvant trials, demonstrating not only enhanced pCR rates over the 60%, but also overall survival benefits [11,12,13,14].
Following these impressive outcomes, the neoadjuvant combination of CT with dual anti-HER2 therapy became the standard of care. However, there remains a significant risk of relapse for these patients.
Despite the extensive variety of clinical trials supporting the efficacy and safety of the neoadjuvant use of pertuzumab, trastuzumab plus CT in HER2-positive BC, these studies often include highly selected patient populations which could not necessarily represent the general population with early-stage HER2-positive BC. Real-world data (RWD) complement clinical trial knowledge by gathering routine clinical practise which encompasses a broader spectrum of patients. Available real-world evidence on the use of this treatment combination under routine clinical practise is still limited [15,16,17], highlighting the need to conduct RWD studies. The NEOPERSUR study aims to confirm whether the improvements in pCR rates after neoadjuvant treatment with dual anti-HER2 therapy for patients with early-stage HER2-positive BC observed in clinical trials are translated into a real-world setting. We also investigated patient and tumour characteristics associated with pCR achievement, pCR prognosis value, and risk factors for BC distant recurrence. Our study will provide further knowledge on pCR after neoadjuvant treatment, and real-life patient characteristics associated with pCR achievement and risk for BC distant relapse. Altogether, this will bring on a step forward to improve neoadjuvant and adjuvant treatment decision-making for these patients.
Methods
Study design and patients
The objectives of the NEOPERSUR study were to investigate the effectiveness of the neoadjuvant dual HER2-blockade with pertuzumab and trastuzumab combined with CT in the achievement of pCR in real-life patients with early-stage HER2-positive BC, and to describe patient characteristics associated with pCR achievement and risk for distant relapse. To accomplish these objectives, we conducted a retrospective medical chart review of patients with early-stage HER2-positive BC who had been treated with neoadjuvant pertuzumab, trastuzumab and CT and subsequent surgery at 13 Spanish hospitals.
Adult women (≥ 18 years) with histologically confirmed HER2-positive localised or locoregionally advanced breast cancer (i.e. 3+ result by immunohistochemistry [IHC], or 2+ result by IHC and positive result by fluorescence in situ hybridization [FISH]), who received neoadjuvant combination treatment with pertuzumab, trastuzumab and chemotherapy (CT), and who had undergone surgery up to December 2018 (with an available anatomopathological report), were included in the study. Patients were excluded if they had received this neoadjuvant combination in the context of a clinical trial or as off-label treatment. Data from the Oncology departments were retrospectively collected from BC diagnosis until December 2022.
This study was approved by the Ethics Committee of the Hospital Universitario Virgen Macarena (Seville, Spain). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice (GCP), and in compliance with European and local requirements. Written informed consent was not required in accordance with the national legislation (Real Decreto 957/2020).
Study endpoints
The primary endpoint was the pCR rate in the breast and axillary lymph nodes (ypT0/Tis ypN0) by local pathology assessment. The pathological response was evaluated using the Miller–Payne grading system [18] as per routine clinical practise. The definition of pCR was the absence of tumour cells of the complete resected breast tumour and axillary lymph nodes, after completion of neoadjuvant systemic therapy. Secondary endpoints included the demographic and clinical characteristics of patients, description of neoadjuvant and adjuvant treatment schemes, distant recurrence rate, distant disease-free survival (D-DFS), overall survival (OS), and toxicity. The OS was estimated as the time elapsed from BC diagnosis to death due to any cause or until database cut-off. The D-DFS was estimated as the free of distant disease interval from breast tumour resection (surgery) until the database cut-off; local relapses were not considered for the analyses.
Statistical analysis
As this was an exploratory study, with a descriptive aim of collecting, summarising and providing data on the treatment of real-life patients with early-stage HER2-positive BC with neoadjuvant pertuzumab, trastuzumab and CT, and the outcomes of this treatment, no pre-specified hypothesis was made, and therefore sample size was not estimated.
A descriptive statistical analysis was performed on the study variables including calculation of measures of central tendency and dispersion (mean and standard deviation [SD], median and interquartile range [IQR]) for quantitative variables, and frequencies and valid percentages for qualitative variables. Data collected from medical records included patients’ age and menopausal status, tumour stage, tumour grade (AJCC cancer staging manual: breast cancer, 8th edition), lymph node involvement, hormone receptor (HR) status (HR-negative or HR-positive) and Ki-67 levels using the 20% cut-off (≤ 20% vs. > 20%) [19]. Comparison between groups with categorical variables was made using the Pearson’s Chi-squared test. Time-to-event endpoint analyses were estimated using the Kaplan–Meier method and compared with the log-rank test.
A logistic regression model was performed to study the association between pCR achievement/relapse and each of the possible clinical factors of interest starting with all variables that were significant in the bivariate analyses (p < 0.200).
Missing data were not considered in the analyses. All hypothesis tests were bilateral; significance was considered at p < 0.05. All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 21.0 (SPSS Inc, Chicago, IL, USA).
Results
Patient characteristics
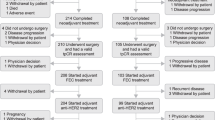
A total of 310 patients diagnosed with early-stage HER2-positive BC, and treated with pertuzumab, trastuzumab plus CT met the selection criteria and were enrolled in the study. The demographic and clinical characteristics of the entire cohort are described in Table 1. Briefly, the mean (SD) age was 51.0 (11.1) years, and 54.5% were premenopausal. Grade 2 and 3 tumours were found in 143 (46.1%) and 90 (29.0%) patients, respectively, and 65.7% of patients had lymph node involvement. Overall, 51% of patients had HR-positive tumours, and 49% of patients had HR-negative disease.
Treatment patterns
In the neoadjuvant setting, pertuzumab and trastuzumab were mostly combined with anthracyclines and taxanes (77.1%), whilst 16.5% of patients received them combined with carboplatin and docetaxel. Only 6.5% of patients received taxane-based chemotherapy, pertuzumab and trastuzumab. Neoadjuvant treatment approaches are shown in Table 2.
The majority (n = 309; 99.7%) of patients had available data on postoperative adjuvant therapy. Trastuzumab was the most common (99.0%), mainly (97.7%) for a period of 12 months, followed by pertuzumab (6.5%), neratinib (1.6%) and the antibody–drug conjugate ado-trastuzumab emtansine (T-DM1; 0.3%) (Table 2).
Effectiveness
Pathological response was evaluable in almost all (n = 307; 99.0%) patients of the study, of whom 62.2% achieved pCR (ypT0/Tis ypN0). According to the neoadjuvant treatments received, pCR was achieved by 66.7% of patients treated with carboplatin and docetaxel combination CT, 62.0% treated with anthracyclines and taxanes, and 52.6% of patients receiving taxane-based CT (Table 3). No significant differences were found between neoadjuvant treatments in terms of pCR achievement (p = 0.555).
Subgroup analyses of response are shown in Table 3. The pCR rate was significantly higher amongst patients with HR-negative disease compared to those with HR-positive tumours (71.1% vs. 53.8%; p = 0.002). In addition, the pCR rate was significantly superior in patients with tumours expressing higher levels of Ki-67 compared to patients with tumours expressing Ki-67 ≤ 20% (62.3% vs. 49.2%; p = 0.021). Achievement of pCR was not significantly associated with the tumour stage nor lymph node involvement.
Multivariate analyses revealed that a HR-negative status and higher levels of Ki-67 (> 20%) were independent predictive factors for achieving pCR in patients with early-stage HER2-positive breast cancer after being treated with the neoadjuvant combination of pertuzumab, trastuzumab and CT (Odds ratio [OR] = 2.143; 95% confidence interval [CI] 1.321–3.477, and OR = 1.931; 95% CI 1.082–3.445, respectively) (Table 3).
At the database cut-off, 43 (13.9%) patients suffered distant relapse, whether central nervous system recurrence (39.5%) or relapsing in other visceral organs or bones (60.5%). Distant recurrence was observed more frequently in those who did not achieve pCR after neoadjuvant therapy (p = 0.001). As displayed in Table 4, 62.8% of patients who did not achieve pCR experienced disease relapse, whilst only 37.2% of patients achieving pCR relapsed. Experiencing distant relapse was not significantly associated with neoadjuvant treatment regimens and neither with receiving adjuvant treatments neratinib and pertuzumab.
The distant relapse rate was significantly higher amongst patients with tumours at more advanced stages (tumour stage I–II: 40.5%; III: 59.5%; p < 0.001). Similarly, patients with lymph node involvement also showed a higher relapse rate than those without affected lymph nodes (85.7% vs. 14.3%; p = 0.003). Distant disease recurrence was not significantly associated with Ki-67 levels or the hormone receptor status (Table 4).
Multivariate analyses exposed the lack of pCR achievement, having tumours in advanced stages (III), and the lymph node affectation as independent predictive factors for distant relapse (OR = 4.206; 95% CI 2.050–8.630, OR = 3.272; 95% CI 1.570–6.823, and OR = 2.730; 95% CI 1.036–7.193, respectively) (Table 4).
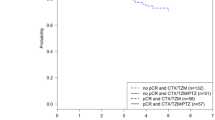
Median overall survival (OS) could not be calculated because more than half of patients were still living. The estimated mean OS time for the entire cohort (n = 309) was 7.5 years (95% confidence interval [CI] 7.3–7.7) (Fig. 1a). According to pathological response, OS was significantly longer in those patients who achieved pCR (7.6 years [95% CI 7.4–7.8] vs. 7.2 years [95% CI: 6.8–7.6]; p = 0.003) (Fig. 1b). Estimated survival at the end of the follow-up was 90.9% (95% CI 84.2–98.1) in patients who achieved pCR versus 75.8% (95% CI 65.3–88) in those who did not.
Similar to OS, median of D-DFS was not reached at the end of the follow-up. Mean D-DFS time for the entire cohort (n = 309) was 7.3 (95% CI 7.1–7.5) years (Fig. 2a). According to pCR achievement, D-DFS was also significantly longer in those patients who achieved pCR (7.4 [95% CI 7.1–7.6] vs. 6.7 [95% CI 6.2–7.2]; p < 0.001) (Fig. 2b). Estimated D-DFS at the end of the follow-up was 89.4% (95% CI 60.3–82.5) in patients who achieved pCR versus 70.6% (95% CI 60.3–82.5) in those who did not.
Toxicity
Overall, 264 (72.9%) patients experienced any adverse event related to chemotherapy, with 14.8% having grade 3–4 toxicities.
Most (n = 273, 88.1%) patients did not experience any toxicity related to anti-HER2 treatment. Grade 3–4 toxicities were reported only in six (1.9%) patients [left ventricular systolic dysfunction (LVSD) in all cases]. None of the patients who experienced cardiac toxicity restarted anti-HER2 treatment.
Discussion
The main goal in the treatment of BC at early stages is to eliminate the tumour and prevent its recurrence. The present study shows that neoadjuvant pertuzumab and trastuzumab combined with CT achieves high pCR rates in real-life patients with early-stage HER2-positive BC. The achievement of pCR was determined by the HR status and Ki-67 levels. Our findings also support the predictive value of pCR in BC distant recurrence after neoadjuvant treatment and postoperative adjuvant therapy. A limited proportion (13.9%) of patients suffered distant relapse. We identified non-pCR achievement, having tumours in advanced stages (III), and the lymph node affectation, as risk factors for BC distant relapse.
In this real-world analysis, we observed a pCR rate of 62.2%, which is similar or even higher than pCR rates reported in previous clinical trials. In the NeoSphere trial, a pCR rate of 45% was observed in patients who received pertuzumab plus trastuzumab and docetaxel [10]. Similar to our analysis, the BERENICE [13] and the GeparSepto [11] trials showed pCR rates > 60% (61.8% and 66.2%, respectively) with neoadjuvant trastuzumab, pertuzumab and anthracycline/taxane-based CT regimens. In addition, the TRAIN-2 trial reported high pCR rates after neoadjuvant CT with or without anthracyclines (67% vs. 68%) plus dual-HER2 blockade [14]. The comparison by CT approaches is limited in clinical trials. Of note, we did not observe differences in pCR achievement according to neoadjuvant treatment patterns.
Although the pCR benefit demonstrated in the clinical trial setting has supported the neoadjuvant use of pertuzumab and trastuzumab with CT for these patients, available data of real-world patients are currently limited. An analysis of the German PRAEGNANT network and other multicentre studies reported slightly reduced pCR rates (52.8% and 46.8%, respectively) [17, 20]. More recently, in a real-life study at two Chinese institutions, Ma et al. observed an overall pCR rate of 64.9% [16], which is in line with our results. Our analysis is also consistent with the Spanish real-world study NEOPETRA (pCR rate of 66%), conducted in a cohort of 250 patients treated with neoadjuvant dual anti-HER2 therapy for early-stage HER2-positive BC [15].
The pCR rate was higher in patients with HR-negative tumours, as exposed in previous neoadjuvant studies [10, 13, 15]. We also found higher levels of Ki-67 (> 20%) as an independent predictive factor for pCR achievement. By contrast, Zhou M. and collaborators associated Ki-67 levels < 15% with pCR in these patients [17]. Although most studies have indicated that a high percentage of Ki-67 correlates to a better response in reducing the size or eliminating the breast tumours, it is still not considered as a validated marker to be used in clinical practise [21], likely because of the retrospective design of these studies and their limited sample size.
Despite of trastuzumab and other anti-HER2 drugs have led to substantial improvements in the outcomes for patients with HER2-positive early-stage BC, there remains a significant risk of recurrence. It is estimated that up to 25% of patients with early-stage HER2-positive BC treated with HER2-targeted therapy will eventually suffer a relapse within 10 years [22]. In our assessment, 13.9% of patients relapsed. Systematic literature reviews include non-achievement of pCR, residual cancer burden, and fewer tumour-infiltrating lymphocytes (TILs), as risk factors specifically identified for early-stage HER2-positive BC recurrence [23]. Differing from them, we found that patients with tumours in advanced stages (III) and the lymph node affectation were risk factors for BC distant relapse in this population. However, our results highlight the importance of the pCR status on the recurrence of the disease. Indeed, other analyses have exposed the pCR as the risk factor most closely associated with decreased risk of HER2-positive early-stage BC recurrence and long-term outcomes [6, 24]. In this regard, predictive tools as the genomic test HER2DX, which efficiently predicts pCR in early-stage HER2-positive BC [25], would be helpful to identify ideal candidates to receive neoadjuvant dual HER2-blockade plus CT to eliminate the tumour and prevent distant recurrence, and in escalating/de-escalating treatments.
Beyond the pCR achievement as a tool to individualise adjuvant systemic therapy, our results from multivariate analyses on the risk factors for BC recurrence unveiled the need of implementing new treatment strategies for patients at high risk of distant relapse. For patients with non-pCR, 14 cycles of T-DM1 have become a new adjuvant recommendation based on the results of the KATHERINE trial [26]. Extrapolation from data of the APHINITY trial hinted that continuing pertuzumab in the adjuvant setting is beneficial for patients with node-positive disease [27]. Otherwise, the phase III ExteNet trial showed benefits of 1-year additional anti-HER2 therapy with neratinib in patients with HR-positive disease previously treated with neoadjuvant trastuzumab-based therapy [28]. In the present study, no patient treated with adjuvant neratinib experienced distant recurrence, but it was administered in less than 2% of patients. Thus, neratinib offers a treatment option in high-risk HR-positive patients. However, Marin A. and collaborators recently showed that acquired secondary mutations in HER2 promote resistance to neratinib [29], bringing out a different strategy to be considered in HER2-mutant BC.
Our data also suggest that dual HER2-blockade with pertuzumab and trastuzumab plus chemotherapy, administered in the neoadjuvant setting, is well tolerated in patients with HER-positive early BC. Anti-HER2 treatment-related toxicities were minimal, and grade 3–4 toxicities were only reported in six patients. All of them experienced LVSD, an expected cardiotoxic effect of trastuzumab and pertuzumab previously documented [30].
Limitations of this study include the absence of a control group and missing information on some variables due to the observational nature of the study. However, most patients had available data for the majority of study parameters. Safety information was limited, and mild or moderate toxicities were not collected. We also lack data on the ECOG performance of analysed patients, and more detailed treatment approaches. Despite these limitations, to our knowledge, this is the most extensive study on the effectiveness and safety of dual HER2-blockade plus chemotherapy in a real-world setting.
In conclusion, this study expands the knowledge on the effectiveness and safety of neoadjuvant pertuzumab and trastuzumab plus CT in real-world patients with early-stage HER2-positive BC and complements clinical trial data. Our results demonstrate that this neoadjuvant combination achieves high pCR rates. The pCR benefit is higher in HR-negative tumours and expressing higher levels of Ki-67. The distant relapse rate was low, and pCR achievement was associated with decreased BC recurrence. In addition, pCR was related to long-term survival outcomes, which remarks the urgent need of identifying novel biomarkers of the pCR. Finally, this neoadjuvant strategy showed an acceptable toxicity profile, with no unexpected safety issues.
Data availability
All data generated during this study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524–41. https://doi.org/10.3322/caac.21754.
Hurvitz SA, Hu Y, O’Brien N, Finn RS. Current approaches and future directions in the treatment of HER2-positive breast cancer. Cancer Treat Rev. 2013;39(3):219–29. https://doi.org/10.1016/j.ctrv.2012.04.008.
EBCTCG. Trastuzumab for early—stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021;22:1139–50. https://doi.org/10.1016/S1470-2045(21)00288-6.
Cortazar P, Geyer CE Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol. 2015;22(5):1441–6. https://doi.org/10.1245/s10434-015-4404-8.
Davey MG, Browne F, Miller N, Lowery AJ, Kerin MJ. Pathological complete response as a surrogate to improved survival in human epidermal growth factor receptor-2-positive breast cancer: systematic review and meta-analysis. BJS Open. 2022;6:zrac028. https://doi.org/10.1093/bjsopen/zrac028.
Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640–7. https://doi.org/10.1016/S1470-2045(14)70080-4.
Untch M, Fasching PA, Konecny GE, Hasmuller S, Lebeau A, Kreienberg R, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29(25):3351–7. https://doi.org/10.1200/JCO.2010.31.4930.
Vivekanandhan S, Knutson KL. Resistance to trastuzumab. Cancers (Basel). 2022;2022(14):5115. https://doi.org/10.3390/cancers14205115.
Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800. https://doi.org/10.1016/S1470-2045(16)00163-7.
Loibl S, Jackisch C, Schneeweiss A, Schmatloch S, Aktas B, Denkert C, et al. Dual HER2-blockade with pertuzumab and trastuzumab in HER2-positive early breast cancer: a subanalysis of data from the randomized phase III GeparSepto trial. Ann Oncol. 2017;28(3):497–504. https://doi.org/10.1093/annonc/mdw610.
Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34. https://doi.org/10.1056/NEJMoa1413513.
Swain SM, Ewer MS, Viale G, Delaloge S, Ferrero JM, Verrill M, et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018;29(3):646–53. https://doi.org/10.1093/annonc/mdx773.
van der Voort A, van Ramshorst MS, van Werkhoven ED, Mandjes IA, Kemper I, Vulink AJ, et al. Three-year follow-up of neoadjuvant chemotherapy with or without anthracyclines in the presence of dual erbb2 blockade in patients with ERBB2-positive breast cancer: a secondary analysis of the TRAIN-2 randomized, Phase 3 trial. JAMA Oncol. 2021;7(7):978–84. https://doi.org/10.1001/jamaoncol.2021.1371.
Gonzalez-Santiago S, Saura C, Ciruelos E, Alonso JL, de la Morena P, Santisteban Eslava M, et al. Real-world effectiveness of dual HER2 blockade with pertuzumab and trastuzumab for neoadjuvant treatment of HER2-positive early breast cancer (The NEOPETRA Study). Breast Cancer Res Treat. 2020;184(2):469–79. https://doi.org/10.1007/s10549-020-05866-1.
Ma X, Zhang X, Zhou X, Ren X, Ma X, Zhang W, et al. Real-world study of trastuzumab and pertuzumab combined with chemotherapy in neoadjuvant treatment for patients with HER2-positive breast cancer. Medicine (Baltimore). 2022;101(40): e30892. https://doi.org/10.1097/MD.0000000000030892.
Zhou M, Wang S, Wan N, Yuan S, Hu X, Zhou W, et al. Efficacy and safety of neoadjuvant pertuzumab plus trastuzumab in combination with chemotherapy regimen in Chinese patients with HER2-positive early breast cancer: a real—world retrospective multi-center cohort study. Ann Transl Med. 2022;10(24):1387. https://doi.org/10.21037/atm-22-6054.
Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–7. https://doi.org/10.1016/s0960-9776(03)00106-1.
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies—improving the management of early breast cancer: St gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26(8):1533–46. https://doi.org/10.1093/annonc/mdv221.
Fasching PA, Hartkopf AD, Gass P, Haberle L, Akpolat-Basci L, Hein A, et al. Efficacy of neoadjuvant pertuzumab in addition to chemotherapy and trastuzumab in routine clinical treatment of patients with primary breast cancer: a multicentric analysis. Breast Cancer Res Treat. 2019;173(2):319–28. https://doi.org/10.1007/s10549-018-5008-3.
Wajid S, Samad FA, Syed AS, Kazi F. Ki-67 and its relation with complete pathological response in patients with breast cancer. Cureus. 2021;13(7): e16788. https://doi.org/10.7759/cureus.16788.
Perez EA, Romond EH, Suman VJ, Jeong J-H, Sledge G Jr, CEG, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–52. https://doi.org/10.1200/jco.2014.55.5730.
O’Shaughnessy J, Gradishar W, O’Regan R, Gadi V. Risk of recurrence in patients with HER2+ early-stage breast cancer: literature analysis of patient and disease characteristics. Clin Breast Cancer. 2023;23(4):350–62. https://doi.org/10.1016/j.clbc.2023.03.007.
Broglio KR, Quintana M, Foster M, Olinger M, McGlothlin A, Berry SM, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016;2(6):751–60. https://doi.org/10.1001/jamaoncol.2015.6113.
Villacampa G, Tung NM, Pernas S, Pare L, Bueno-Muino C, Echavarria I, et al. Association of HER2DX with pathological complete response and survival outcomes in HER2-positive breast cancer. Ann Oncol. 2023;34(9):783–95. https://doi.org/10.1016/j.annonc.2023.05.012.
von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28. https://doi.org/10.1056/NEJMoa1814017.
Piccart M, Procter M, Fumagalli D, de Azambuja E, Clark E, Ewer MS, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol. 2021;39(13):1448–57. https://doi.org/10.1200/JCO.20.01204.
Chan A, Moy B, Mansi J, Ejlertsen B, Holmes FA, Chia S, et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin Breast Cancer. 2021;21(1):80-91 e7. https://doi.org/10.1016/j.clbc.2020.09.014.
Marin A, Mamun AA, Patel H, Akamatsu H, Ye D, Sudhan DR, et al. Acquired secondary HER2 mutations enhance HER2/MAPK signaling and promote resistance to HER2 kinase inhibition in breast cancer. Cancer Res. 2023. https://doi.org/10.1158/0008-5472.CAN-22-3617.
Alhussein MM, Mokbel A, Cosman T, Aghel N, Yang EH, Mukherjee SD, et al. Pertuzumab Cardiotoxicity in patients with HER2-positive cancer: a systematic review and meta-analysis. CJC Open. 2021;3(11):1372–82. https://doi.org/10.1016/j.cjco.2021.06.019.
Acknowledgements
Scientific advisory and medical writing assistance were provided by Carla Martín Cortázar from Evidenze Health España S.L. during the preparation of this manuscript, and were funded by Roche España S.A.
Funding
Scientific advisory and medical writing assistance services were funded by Roche España S.A.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Alejandro Falcón González, Manuel Ruiz Borrego, and Eloísa Rubio Pérez. The first draft of the manuscript was written by Alejandro Falcón González and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr A. Falcón González has received research speaker honoraria from Roche, AstraZeneca, Daiichi, Eisai, Gilead, Seagen, Pfizer, Novartis, Pierre Fabre, Grünenthal and Lilly; and has received consultant honoraria from AstraZeneca, Pfizer, Daiichi, Seagen and Esteve. Dr J. Cruz Jurado has received speaker honoraria from Glaxo, AstraZeneca, Roche, Novartis, Pharmamar, Eisai, Lilly, Daichii Sankyo, Gilead, Seagen, MSD, Pierre Fabre, and Pfizer; has conducted consultant/advisory role at AstraZeneca, Roche, Novartis, Pharmamar, Eisai, Lilly, Glaxo, Daichii Sankyo, Gilead, Seagen, Deciphera, and Pfizer; and has received travel support from Novartis, Gilead, Pharmamar, and Daichii-Sankyo. Dr E. Llabrés Valentí has received research speaker honoraria from Bristol, Ipsen, AstraZeneca, Daichii, Pfizer, Gilead, Novartis and Lilly; and has received consultant honoraria from AstraZeneca, Daichii and Seagen. Dr R. Urbano Cubero has received research speaker honoraria from Pfizer, Novartis, Pierre Fabre, and Lilly. Dr MC. Álamo de la Gala has received research speaker honoraria from Roche, Pfizer, Novartis, Pierre Fabre, Bristol-Myers Squibb. Dr MA. Martinez Guisado has received research speaker honoraria from Roche, Daiichi, Gilead, Seagen, Pfizer, Novartis, Pierre Fabre, and Lilly; and has received consultant honoraria from Pfizer and Daiichi. Dr CJ. Rodríguez González has received research speaker honoraria from AstraZeneca, Eisai, MSD, GSK, Pfizer, Novartis and Lilly. Dr M. Amérigo Góngora has received funding for speaker bureau from Kyowa Kirin and Grünenthal. Dr P. López Álvarez has received research speaker honoraria from Roche, AstraZeneca, Daichii, Eisai, Pfizer, Novartis, Pierre Fabre, Amgen, Celgene and Lilly; and has received consultant honoraria from AstraZeneca, Daichii, Roche, Novartis, Celgene and Pfizer. Dr P. Sánchez Rovira has received research speaker honoraria from Roche, AstraZeneca, Pierre-Fabre Gilead, Seagen, Pfizer, Novartis, and Lilly; has received consultant honoraria from AstraZeneca, Roche, Pfizer, Novartis, and Seagen; and has received research funds from Roche and AstraZeneca. Dr E. González Flores has received research speaker honoraria from Roche, Daiichi, Gilead, Seagen, Pfizer, Novartis, and Lilly; and has received consultant honoraria from AstraZeneca, Pfizer, Novartis, Daiichi and Seagen. Dr F. Henao Carrasco has received research speaker honoraria from Roche, Daiichi, Gilead, Pfizer, Novartis, Eisai and Lilly; and has received consultant honoraria from AstraZeneca, Pfizer, Novartis, Lilly, Pierre Fabre, Daiichi and Seagen. Dr M. Valero Arbizu has received speaker grants from Pfizer, Novartis, Roche, MSD, Seagen, Pierre Fabre, Eisai, and Sanofi-Aventis; and has received research fund from Quironsalud. Dr A. Quílez Cutillas has received research speaker honoraria from Roche, AstraZeneca, Bristol, Pfizer, Novartis, Pierre Fabre, Clovis and Grunenthal; and has received consultant honoraria from GSK, Pharmamar and Clovis. Dr J. Salvador Boffil has received research speaker honoraria from Roche, AstraZeneca, Daiichi, Gilead, Pfizer, Novartis, and Lilly; and has received consultant honoraria from Pfizer, Daiichi, Roche, and Novartis. Dr M. Ruiz-Borrego has received speaker grants from AstraZeneca, Daiichi, Novartis, Pfizer, Gilead, Pierre Fabre; has received the advisory board honoraria from AstraZeneca, Daiichi, Pierre Fabre, Gilead; has received research funding from Pfizer; and has received travel support from Novartis, Roche, and Pfizer. The rest of authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the Ethics Committee of the Hospital Universitario Virgen Macarena (Seville, Spain). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice (GCP), and in compliance with European and local requirements. Written informed consent was not required in accordance with the national legislation (Real Decreto 957/2020).
Consent to publish
Not applicable as this manuscript does not contain any individual personal data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Falcón González, A., Cruz Jurado, J., Llabrés Valenti, E. et al. Real-world experience with pertuzumab and trastuzumab combined with chemotherapy in neoadjuvant treatment for patients with early-stage HER2-positive breast cancer: the NEOPERSUR study. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03440-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12094-024-03440-5