Abstract
Objective
This study aimed to determine whether the combined use of bevacizumab could improve overall survival (OS) in patients with brain metastasis (BM), epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC) undergoing cerebral radiotherapy.
Materials and methods
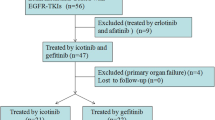
A total of 237 patients with EGFR-mutant lung adenocarcinoma and BM met the inclusion criteria for this retrospective study, including 102 patients in the bevacizumab treatment group and 135 in the non-bevacizumab group. The Kaplan–Meier method was used for survival analysis. Univariate and multivariate analyses were performed to identify EGFR-mutated BM prognostic factors for these patients.
Results
At the end of the last follow-up period, 176 patients (74.3%) had died, and the median overall survival (OS) was 34.2 months. We observed a significant difference in the median OS between the bevacizumab and non-bevacizumab groups (45.8 months vs 30.0 months, P < 0.0001). Among the 178 (75.1%) patients who received cerebral radiotherapy, the median OS of patients in the bevacizumab + cerebral radiotherapy group was 45.8 months versus 32.0 months in the non-bevacizumab + cerebral radiotherapy group, respectively (P = 0.0007). Patients treated with bevacizumab after cerebral radiotherapy had a longer median OS than patients treated with bevacizumab before cerebral radiotherapy (59.4 months vs 33.7 months, P = 0.0198). In the univariate analysis, smoking status, Lung-molGPA scores, and bevacizumab therapy showed correlations (HR = 1.450, P = 0.045; HR = 0.700, P = 0.023; HR = 0.499, P < 0.001). Multivariate analysis showed that bevacizumab therapy alone (hazard ratio [HR] = 0.514; P < 0.001) was independently associated with improved OS.
Conclusion
In patients with BM from EGFR-mutated NSCLC, cerebral radiotherapy with bevacizumab markedly improved OS. This improvement was more evident after cerebral radiotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastasis (BM) remains one of the most common distant metastases in patients with advanced non-small cell lung cancer (NSCLC) and is associated with poor prognostic outcomes [1, 2]. Accumulating evidence has shown that EGFR gene mutation-positive NSCLC patients are prone to BM, with frequencies ranging from 44 to 63% [3]. EGFR tyrosine kinase inhibitors (TKI) have shown certain therapeutic effects on BM in patients with EGFR mutations; however, intracranial progression-free survival (iPFS) is only 8–10 months and almost all patients experience disease progression [4, 5]. Cerebral radiotherapy is an effective method that plays a key role in treating BM [6]. These studies confirmed that cerebral radiotherapy improves the effectiveness of EGFR-TKI therapy by disrupting the blood–brain barrier (BBB), especially for NSCLC patients with NSCLC with BM and EGFR mutations. Cerebral radiotherapy can reduce recurrence and improve overall survival (OS) [7, 8]. However, the survival benefits come at the expense of neurocognitive toxicity. Therefore, researchers are increasingly concerned about the potential long-term effects of cerebral radiotherapy on neurocognitive function and quality of life [9].
Bevacizumab (Avastin), a humanized monoclonal antibody, works by competing with vascular endothelial growth factor (VEGF) for VEGF receptors (Flt-1 and KDR) [10,11,12]. High levels of VEGF expression cause abnormal neovascularization, leading to increased vascular permeability and damage to the BBB, which are crucial for the formation of BM in lung adenocarcinoma [13]. The VEGF inhibitor bevacizumab effectively inhibited this process. Bevacizumab plays a role in pruning blood vessels, regulating vascular permeability, and normalizing vasculature [14]. A phase 3 trial (NEJ026) conducted in untreated NSCLC patients with EGFR mutations who were randomly assigned to receive either erlotinib plus bevacizumab combination therapy or erlotinib monotherapy showed that bevacizumab plus EGFR-TKI as a first-line treatment for patients with advanced non-squamous EGFR-mutant NSCLC significantly prolonged progression-free survival (PFS) [15].
Bevacizumab was also demonstrated to be effective in BM, with an overall intracranial response rate of 61.2% in the BRAIN study [16]. However, few studies have reported the potential effects of bevacizumab in patients with EGFR-mutant NSCLC who develop BM, especially in those who received both EGFR-TKI treatment and cerebral radiotherapy. A retrospective study analyzed 49 patients with NSCLC-BM, including 21 treated with bevacizumab combined with whole-brain radiotherapy plus stereotactic radiosurgery (WBRT-SRS) and 28 treated with WBRT-SRS alone. The results showed that bevacizumab combined with radiotherapy improved the overall efficacy of BM in patients with NSCLC and reduced peritumoral edema in the BM [17]. Notably, the conclusions from this trial were limited by the relatively small sample sizes and have not been well investigated in patients with EGFR mutations. Therefore, the efficacy and safety of bevacizumab combined with cerebral radiotherapy warrant further investigation.
This study aimed to determine whether bevacizumab could improve OS after cerebral radiotherapy in patients with EGFR-mutant NSCLC who developed BM.
Materials and methods
Patients
This study was approved by the Ethics Review Committee of Shandong Cancer Hospital (Ethical Approval Number: 2021003002) and conducted in accordance with the Declaration of Helsinki. In this retrospective study, the vast majority of patients signed the informed consent form when they were alive, while only a few deceased patients did not get the informed consent form at the time of the study.
We screened patients with EGFR-mutant lung adenocarcinoma and BM who were hospitalized at Shandong Cancer Hospital between September 2008 and September 2020. The selection criteria were as follows:(1) NSCLC with EGFR-sensitive mutations at first diagnosis, (2) enhanced computed tomography (CT) or magnetic resonance imaging (MRI) for the diagnosis of BM, (3) detailed clinical information, including treatment choice and clinicopathological features, (4) administration of EGFR-TKI (e.g., gefitinib, erlotinib, or osimertinib), (5) no other primary malignant neoplasms, and (6) complete follow-up data.
The clinicopathological characteristics included age, sex, smoking history, EGFR mutation status, type of EGFR-TKI, BM during initial diagnosis and treatment, and initiation of bevacizumab treatment. Recent follow-up visits and deaths were also recorded. OS was determined from the date of pathological diagnosis of lung adenocarcinoma to the date of death from any cause or the last survival follow-up.
The Lung-molGPA is a specifically graded prognostic assessment based on patient age, Karnofsky performance status (KPS), extracranial and BM counts, and mutation status. Score criteria are shown in Table 1 [18, 19].
Treatment
Patients received bevacizumab in combination with EGFR-TKI or chemotherapy as first-, second-, or more than second-line treatment. Bevacizumab was administered as maintenance therapy until disease progression, unacceptable toxicity, or loss of clinical benefit.
Cerebral radiotherapy included whole-brain radiotherapy (WBRT), local radiotherapy, and WBRT + Boost. The detailed radiotherapy treatment plans are shown in Supplementary Table 1.
Statistical methods
The Chi-square test was used to compare patient characteristics between the two groups. The Kaplan–Meier method was used for survival analysis, and the log-rank test was used to test the influence of individual variables on survival. Univariate and multivariate Cox regression analyses were performed to examine the association between clinical factors and OS. A value of P < 0.05 was considered statistically significant, and variables with significance in univariate analyses were selected for multivariate analysis. All analyses were performed using SPSS Version25.0.
Results
Patient characteristics
A total of 237 patients with EGFR-mutant lung adenocarcinoma and BM met the inclusion criteria for this retrospective study, including 102 in the bevacizumab group and 135 in the non-bevacizumab group. Detailed patient characteristics are shown in Table 2. The median age of the patients at diagnosis was 53 years (range 28–81 years). The majority of patients were women (152,64.1%) and non-smokers (189,79.7%). According to the Lung-molGPA classification system, the numbers of patients with scores 1–2 and 2.5–4 were 85 (35.9%) and 150 (63.3%), respectively. Regarding treatment modalities, all patients received EGFR TKIs treatment, with 178 (75.1%) receiving cerebral radiotherapy.
Survival analysis of the whole cohort in this study
At the end of the last follow-up period, 176 patients (74.3%) had died. The median OS of all patients was 34.2 months (Fig. 1A). The median OS between the groups with mutations in exons 19 and 21 was 34.2 months and 32.5 months, respectively (log-rank, P = 0.4882; Fig. 1B). The median OS of the smoking and never smoking groups was 32.1 months and 36.0 months, respectively (log-rank, P = 0.0371; Fig. 1C). We also compared survival differences between groups stratified by the Lung-molGPA score, which affects OS in patients with NSCLCs with BMs. There was a trend of different OS between groups with Lung-molGPA scores 1–2 and 2.5–4 (28.4 months vs 37.1 months, P = 0.0205; HR: 1.457; 95% CI 1.060 to 2.003; Fig. 1D). There was no significant difference in OS between patients who received cerebral radiotherapy and those who did not receive cerebral radiotherapy (median OS, 35.6 months vs 30.7 months, P = 0.2286; Fig. 1E).
Effect of bevacizumab on overall survival
This study aimed to verify whether bevacizumab is a promising candidate for patients with EGFR-mutant NSCLC who develop BM. The median OS of patients who received bevacizumab was significantly longer than that of patients who did not receive bevacizumab (45.8 months vs 30.0 months, P < 0.0001; Fig. 1F).
For patients stratified by Lung-molGPA scores of 1–2, the median OS based on patients with- or without-bevacizumab treatment was 33.7 and 26.6 months, respectively (P = 0.0574, Fig. 2A). For patients stratified by Lung-molGPA scores of 2.5–4, the median OS based on patients with- or without-bevacizumab treatment was 46.6 and 27.5 months, respectively (P = 0.0002, Fig. 2B).
The OS benefit of bevacizumab treatment in the low Lung-molGPA (A) and high Lung-molGPA (B) groups. In patients with BM from EGFR-mutated NSCLC, the addition of bevacizumab to cerebral radiotherapy improved OS (C), an improvement that was more evident after cerebral radiotherapy (D). There is also a comparison of OS between cerebral and non-cerebral radiotherapy groups of patients with (E) and without (F) bevacizumab treatment. EGFR epidermal growth factor receptor, BM brain metastasis, OS, overall survival, NSCLC non-small cell lung cancer
Cerebral radiotherapy addition of bevacizumab was markedly associated with a longer OS
Among patients who received cerebral radiotherapy, we observed a significant difference in median OS between the bevacizumab and non-bevacizumab group (45.8 months vs 27.1 months, P < 0.001; Fig. 2C). Our analysis suggested that patients treated with bevacizumab after cerebral radiotherapy had a longer median OS than patients treated with bevacizumab before cerebral radiotherapy (59.4 months vs 33.7 months, P = 0.0198; Fig. 2D), indicating that this may be the optimal time for bevacizumab addition.
The median OS of patients in the bevacizumab + cerebral radiotherapy group was 45.8 months versus 45.3 months in the bevacizumab + non-cerebral radiotherapy group (P = 0.8937; Fig. 2E). However, in the groups of patients without bevacizumab, there was a significant OS benefit for cerebral radiotherapy versus non-cerebral radiotherapy (32.0 months vs 21.6 months, P = 0.0475; Fig. 2F).
Univariable and multivariable analyses of covariable with the OS results
Univariate and multivariate Cox models were used to analyze and evaluate the influence of different variables, including sex, age (≥ 60 years or not), smoking status, Lung-molGPA scores, bevacizumab treatment, cerebral radiotherapy, concurrent cerebral radiotherapy, and EGFR-TKI on the survival rate. In the univariate analysis, smoking status, Lung-molGPA scores, and bevacizumab treatment showed significant correlations and trends (HR = 1.450, P = 0.045; HR = 0.700, P = 0.023; HR = 0.499, P < 0.001, respectively). The remaining variables were not significant. Multivariate analysis showed that bevacizumab therapy alone (hazard ratio [HR] = 0.514; P < 0.001) was independently associated with an improvement (Table 3).
Discussion
Our retrospective study demonstrated that bevacizumab treatment is associated with superior OS in patients with BM and EGFR-mutant NSCLC. The median OS of the bevacizumab-treated group was 45.8 months compared to 30 months in the non-bevacizumab-treated group. Jiang et al. found that bevacizumab plus EGFR-TKI significantly prolonged both PFS and OS in patients with EGFR-mutant NSCLC and multiple BM [20]. Recently, a real-world study using the SEER-Medicare dataset also observed that bevacizumab resulted in a 25% reduction in the risk of death in NSCLC patients with synchronous BM compared to patients without intracranial disease (HR: 0.75, P = 0.020) [21]. In the JO25567 and NEJ026 trials, bevacizumab combined with systemic therapy showed significantly prolonged PFS, but no OS benefit [15, 22]. We found that BM patient populations were excluded from the JO25567 study and comprised only 32% of the overall population in the NEJ026 study, which may be associated with the negative OS results in these studies. Comparatively, all the patients included in our study had BM. Overall, all these studies demonstrated that patients with BM represent a population that shows major bevacizumab benefits. This may be because extensive neoangiogenesis plays an important role in the formation of BM from lung adenocarcinoma. Inhibition of this early angiogenic switch can arrest BM growth [23, 24]. Therefore, bevacizumab may effectively inhibit BM progression in patients with NSCLC.
Cerebral radiotherapy is widely used as the standard treatment for BMs. It is one of the main tools for increasing local control in patients with EGFR-mutant NSCLC-BM [6, 25, 26]. In a previous study, we showed that EGFR-TKI combined with craniocerebral radiotherapy improved iPFS, OS and PFS in patients with EGFR-mutant lung adenocarcinoma with BM [27]. The current study further explored whether the combined use of bevacizumab could lead to survival benefits in patients with EGFR-mutant NSCLC-BM undergoing cerebral radiotherapy. Our results showed that bevacizumab combined with cerebral radiotherapy significantly prolonged OS in EGFR-mutant NSCLC patients with BM compared with non-bevacizumab treatment. Another retrospective study showed that compared with gefitinib-WBRT and WBRT alone, bevacizumab–gefitinib-WBRT significantly improved the PFS and OS of BM patients (P < 0.05) and was ranked as the most effective treatment because it had the highest response rate (RR) and disease control rate (DCR) [28]. This finding is consistent with our conclusions. These survival benefits may be due to the potential synergistic effects of radiation and bevacizumab. Radiation causes vasogenic edema, ischemia, and hypoxia. Subsequently, tissue hypoxia increases the expression of hypoxia-inducible factor (HIF)-1. Elevated HIF-1α expression stimulates reactive astrocytes and endothelial cells to secrete the pro-angiogenic factor VEGF. High levels of VEGF expression allow plasma proteins to leak into the cerebral parenchyma, leading to brain edema development [28, 29]. Bevacizumab blocks radiation-induced increased VEGF expression and mediates the normalization of tumor blood vessels, thus reducing brain edema. Moreover, bevacizumab may be useful for radiotherapy treatment. When tumor cells are in the radio-insensitive phase of the cell cycle, bevacizumab can restrict their proliferation by restricting abnormal vascular regeneration, thereby supplementing radiation limitations and achieving a synergistic effect of inhibiting tumors [30,31,32]. Thus, bevacizumab in combination with cerebral radiotherapy complements each other in the treatment of patients with BM. This mechanism provides a theoretical basis for validating the clinical benefits of this combination.
We further found that the benefit of bevacizumab combined with cerebral radiotherapy may be facilitated by the optimal treatment timing. Our study showed that patients treated with bevacizumab after cerebral radiotherapy had a longer median OS than patients treated with bevacizumab before cerebral radiotherapy, which might be related to bevacizumab’s alleviation of radiation brain necrosis (RN) caused by cerebral radiotherapy. RN is a serious complication that occurs anywhere from 3 months to several years after patients receive radiation therapy [33, 34]. RN occurs in 3–24% of patients with BM and is characterized by headaches, confusion, dizziness, memory loss, personality changes, and seizures, which have a serious impact on the patient’s quality of life [35, 36]. RN is associated with increased BBB permeability, inflammation, and elevated VEGF levels [37]. Bevacizumab counteracted the effects of VEGF on the RN when administered after cerebral RT. Gonzalez was first to report the use of bevacizumab treatment for RN in 2007 [38]. A placebo-controlled, double-blind study evaluated the safety and efficacy of bevacizumab in patients with symptomatic and progressive RN. Patients who received bevacizumab showed MRI responses and improvements in neurological signs and symptoms at 6 weeks. However, patients receiving the placebo had worsening neurological signs, symptoms, and progression of RN on MRI at 6 weeks [39]. Therefore, regular follow-up examinations are important to determine the response to bevacizumab in patients with RN after cerebral radiotherapy. However, most patients in our study did not undergo systematic clinical visits with MRI. The therapeutic decision to use bevacizumab after cerebral radiotherapy requires further support from long-term follow-up studies, although OS benefits were observed in our study.
Our study has some limitations. First, considering the retrospective nature of this study, it is subject to inherent limitations. Second, most patients in our study did not undergo a systematic clinical follow-up through MRI. Therefore, a long-term follow-up study with a large population is required to confirm the mechanism underlying the effects of bevacizumab treatment in patients with BM after cerebral radiotherapy.
Conclusion
Bevacizumab significantly improved the OS of patients with BM who underwent cerebral radiotherapy, and this benefit was even greater after cerebral radiotherapy.
Data availability
Data are available on request to the authors.
Abbreviations
- BBB:
-
Blood–brain barrier
- BM:
-
Brain metastasis
- CT:
-
Computed tomography
- EGFR:
-
Epidermal growth factor receptor
- KPS:
-
Karnofsky performance status
- MRI:
-
Magnetic resonance imaging
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- RN:
-
Radiation brain necrosis
- RR:
-
Response rate
- TKI:
-
Tyrosine kinase inhibitors
- VEGF:
-
Vascular endothelial growth factor
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Sosa E, D’Souza G, Akhtar A, Sur M, Love K, Duffels J, et al. Racial and socioeconomic disparities in lung cancer screening in the United States: a systematic review. CA Cancer J Clin. 2021;71:299–314.
Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, et al. Brain metastases in patients with EGFR -mutated or ALK -rearranged non-small-cell lung cancers. Lung Cancer. 2015;88:108–11.
Cho JH, Lim SH, An HJ, Kim KH, Park KU, Kang EJ, et al. Osimertinib for patients with non–small-cell lung cancer harboring uncommon EGFR mutations: a multicenter, open-label, phase II trial (KCSG-LU15-09). J Clin Oncol. 2020;38:488–95.
Ballard P, Yates JWT, Yang Z, Kim D-W, Yang JC-H, Cantarini M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–40.
Brown PD, Ahluwalia MS, Khan OH, Asher AL, Wefel JS, Gondi V. Whole-brain radiotherapy for brain metastases: evolution or revolution? J Clin Oncol. 2018;36:483–91.
Martínez P, Mak RH, Oxnard GR. Targeted therapy as an alternative to whole-brain radiotherapy in EGFR -mutant or ALK -positive non-small-cell lung cancer with brain metastases. JAMA Oncol. 2017;3:1274.
Wang C, Lu X, Lyu Z, Bi N, Wang L. Comparison of up-front radiotherapy and TKI with TKI alone for NSCLC with brain metastases and EGFR mutation: a meta-analysis. Lung Cancer. 2018;122:94–9.
Pellerino A, Bruno F, Rudà R, Soffietti R. Systemic therapy for lung cancer brain metastases. Curr Treat Options Oncol. 2021;22:110.
Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer. 2019;18:21.
Le X, Nilsson M, Goldman J, Reck M, Nakagawa K, Kato T, et al. Dual EGFR-VEGF pathway inhibition: a promising strategy for patients with EGFR-mutant NSCLC. J Thorac Oncol. 2021;16:205–15.
Akamatsu H, Toi Y, Hayashi H, Fujimoto D, Tachihara M, Furuya N, et al. Efficacy of osimertinib plus bevacizumab vs osimertinib in patients with EGFR T790M–mutated non-small cell lung cancer previously treated with epidermal growth factor receptor-tyrosine kinase inhibitor. JAMA Oncol. 2021;7:386–94.
Masuda C, Sugimoto M, Wakita D, Monnai M, Ishimaru C, Nakamura R, et al. Bevacizumab suppresses the growth of established non-small-cell lung cancer brain metastases in a hematogenous brain metastasis model. Clin Exp Metastasis. 2020;37:199–207.
Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017.
Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–35.
Besse B, Le Moulec S, Mazières J, Senellart H, Barlesi F, Chouaid C, et al. Bevacizumab in patients with nonsquamous non-small cell lung cancer and asymptomatic, untreated brain metastases (BRAIN): A Nonrandomized Phase II Study. Clin Cancer Res. 2015;21:1896–903.
Li L, Feng M, Xu P, Wu YL, Yin J, Huang Y, et al. Stereotactic radiosurgery with whole brain radiotherapy combined with bevacizumab in the treatment of brain metastases from NSCLC. Int J Neurosci. 2023;133(3):334–41.
Deng G, Zhang Y, Ke J, Wang Q, Qin H, Li J, et al. Effect of brain radiotherapy strategies on prognosis of patients with EGFR-mutant lung adenocarcinoma with brain metastasis. J Transl Med. 2021;19:486.
Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40:331–57.
Jiang T, Zhang Y, Li X, Zhao C, Chen X, Su C, et al. EGFR-TKIs plus bevacizumab demonstrated survival benefit than EGFR-TKIs alone in patients with EGFR-mutant NSCLC and multiple brain metastases. Eur J Cancer. 2019;121:98–108.
Ascha MS, Wang JF, Kumthekar P, Sloan AE, Kruchko C, Barnholtz-Sloan JS. Bevacizumab for the treatment of non-small cell lung cancer patients with synchronous brain metastases. Sci Rep. 2019;9:17792.
Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–44.
Liang P, Wang Y-D, Wei Z-M, Deng Q-J, Xu T, Liu J, et al. Bevacizumab for non-small cell lung cancer patients with brain metastasis: a meta-analysis. Open Med. 2020;15:589–97.
Watanabe H, Ichihara E, Kayatani H, Makimoto G, Ninomiya K, Nishii K, et al. VEGFR2 blockade augments the effects of tyrosine kinase inhibitors by inhibiting angiogenesis and oncogenic signaling in oncogene-driven non-small-cell lung cancers. Cancer Sci. 2021;112:1853–64.
Miyawaki E, Kenmotsu H, Mori K, Harada H, Mitsuya K, Mamesaya N, et al. Optimal sequence of local and EGFR-TKI therapy for EGFR-mutant non-small cell lung cancer with brain metastases stratified by number of brain metastases. Int J Radiat Oncol. 2019;104:604–13.
Schiff D, Messersmith H, Brastianos PK, Brown PD, Burri S, Dunn IF, et al. Radiation therapy for brain metastases: ASCO guideline endorsement of ASTRO guideline. J Clin Oncol. 2022;40:2271–6.
Deng G, Tan X, Li Y, Zhang Y, Wang Q, Li J, et al. Effect of EGFR-TKIs combined with craniocerebral radiotherapy on the prognosis of EGFR-mutant lung adenocarcinoma patients with brain metastasis: a propensity-score matched analysis. Front Oncol. 2023;13:1049855.
Yang RF, Yu B, Zhang RQ, Wang XH, Li C, Wang P, et al. Bevacizumab and gefitinib enhanced whole-brain radiation therapy for brain metastases due to non-small-cell lung cancer. Braz J Med Biol Res. 2018;51:e6073.
Baumann J, Tsao C-C, Patkar S, Huang S-F, Francia S, Magnussen SN, et al. Pericyte, but not astrocyte, hypoxia inducible factor-1 (HIF-1) drives hypoxia-induced vascular permeability in vivo. Fluids Barriers CNS. 2022;19:6.
Zhuang H-Q, Sun J, Yuan Z-Y, Wang J, Zhao L-J, Wang P, et al. Radiosensitizing effects of gefitinib at different administration times in vitro. Cancer Sci. 2009;100:1520–5.
Tsien CI, Pugh SL, Dicker AP, Raizer JJ, Matuszak MM, Lallana EC, et al. NRG oncology/RTOG1205: a randomized phase II trial of concurrent bevacizumab and reirradiation versus bevacizumab alone as treatment for recurrent glioblastoma. J Clin Oncol. 2023;41:1285–95.
Rodríguez-Camacho A, Flores-Vázquez JG, Moscardini-Martelli J, Torres-Ríos JA, Olmos-Guzmán A, Ortiz-Arce CS, et al. Glioblastoma treatment: state-of-the-art and future perspectives. Int J Mol Sci. 2022;23:7207.
Torcuator R, Zuniga R, Mohan YS, Rock J, Doyle T, Anderson J, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009;94:63–8.
Tijtgat J, Calliauw E, Dirven I, Vounckx M, Kamel R, Vanbinst AM, et al. Low-dose bevacizumab for the treatment of focal radiation necrosis of the brain (fRNB): a single-center case series. Cancers. 2023;15:2560.
Lee D, Riestenberg RA, Haskell-Mendoza A, Bloch O. Brain metastasis recurrence versus radiation necrosis. Neurosurg Clin N Am. 2020;31:575–87.
Yang X, Ren H, Fu J. Treatment of radiation-induced brain necrosis. Oxid Med Cell Longev. 2021;2021:1–15.
Wang Y, Wang E, Pan L, Dai J, Zhang N, Wang X, et al. A new strategy of CyberKnife treatment system based radiosurgery followed by early use of adjuvant bevacizumab treatment for brain metastasis with extensive cerebral edema. J Neurooncol. 2014;119:369–76.
Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol. 2007;67:323–6.
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol. 2011;79:1487–95.
Funding
This work was supported by Taishan Scholars Program of Shandong Province (No. ts20190982); the National Natural Science Foundation of China (Grant No.81902355).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. Yuanliang Zhou performed the statistical analysis and wrote the paper draft. Guangchuan Deng, Hongyue Qin, and Qi Wang collected the clinical information. Jingchao Li and Yankang Li analyzed and interpreted data. Jianbin Li and ZhenXiang Li designed the study together.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
This study was approved by Ethics Review Committee of Shandong Cancer Hospital, Ethical Approval Numbers/ID: 2021003002.
Consent for publication
The need for informed consent was waived due to the retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianbin Li is the main corresponding author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Y., Li, J., Li, Y. et al. Bevacizumab improved prognosis for advanced EGFR-mutant lung adenocarcinoma with brain metastasis receiving cerebral radiotherapy. Clin Transl Oncol 26, 1968–1975 (2024). https://doi.org/10.1007/s12094-024-03418-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-024-03418-3