Abstract
Purpose
This study aimed to validate the classification of breast cancer (BC) patients in progression risk groups based on total tumor load (TTL) value to predict lymph node (LN) affectation after neo-adjuvant systemic therapy (NAST) obtained in the NEOVATTL study.
Methods/patients
This was an observational, retrospective, international, multicenter study including patients with infiltrating BC who received NAST followed by sentinel lymph node biopsy (SLNB) analyzed with one-step nucleic acid amplification (OSNA) from nine Spanish and two Italian hospitals. Patients were classified into three groups according to the progression risk, measured as disease-free survival (DFS), based on TTL values (> 250, 250–25,000, and > 25,000 copies/μL). The previous (NEOVATTL study) Cox regression model for prognosis was validated using prognostic index (PI) and Log ratio test (LRT) analyses; the value of TTL for axillary non-SLN affectation was assessed using receiver operating characteristic (ROC) curves.
Results
We included 263 patients with a mean age of 51.4 (± SD 10.5) years. Patients with TTL > 25,000 copies/μL had a shorter DFS (HR 3.561 [95% CI 1.693−7.489], p = 0.0008 vs. TTL ≤ 25,000). PI and LRT analyses showed no differences between the two cohorts (p = 0.2553 and p = 0.226, respectively). ROC analysis showed concordance between TTL and non-SLN involvement (area under the curve 0.828), with 95.7% sensitivity and 92.9% specificity at a TTL cut-off of > 15,000 copies/μL.
Conclusions
In BC patients who had received NAST and underwent SLNB analysis using OSNA, a TTL value of > 25,000 copies/μL was associated with a higher progression risk and > 15,000 copies/μL was predictive of non-SLN involvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neo-adjuvant systemic treatment (NAST) is currently being used as a preoperative treatment modality in early-stage breast cancer (BC), offering the opportunity to further de-escalate the surgical management of the axilla in patients undergoing BC surgery [1,2,3,4]. However, there is still a lack of consensus regarding the post-treatment surgical approach to axillary lymph node dissection (ALND).
Sentinel lymph node biopsy (SLNB) has been established as the gold standard for pathologic evaluation of the axilla in patients with operable BC [5]. However, attitudes upon finding metastatic disease in sentinel lymph nodes (SLN) post-NAST vary widely in clinical practice, with a trend toward a conservative approach. Currently, international consensuses recommend lymphadenectomy for any type of metastatic involvement and many clinical guidelines still lack recommendations for post-NAST SLN [6,7,8].
The diagnostic performance of SLNB by conventional histopathology after NAST remains controversial as this treatment could limit the accuracy of the nodal histological evaluation [9,10,11]. Cytokeratin 19 (CK19) is a membrane protein expressed by most BC even post-NAST, constituting a good biomarker to detect metastases [12, 13]. One-step nucleic acid amplification (OSNA) is a molecular technique that detects the messenger ribonucleic acid (mRNA) copy number of CK19 present in SLN. OSNA enables calculating the total tumor burden (TTL) in an automated and reproducible manner, providing objective information about the metastatic burden present in lymph nodes (LNs) [14], detecting micro- and macro-metastases of LNs with high sensitivity and specificity [15, 16]. Therefore, its use could provide more sensitive information regarding the LNs metastatic status post-NAST compared to conventional histological examination, limiting overtreatment or unnecessary ALND and adding more precision and reproducibility to axillary surgical management [9, 17].
The PLUTTO study demonstrated TTL's clinical value in BC patients without NAST, correlated TTL with disease-free survival (DFS), and identified low- and high-risk progression groups based on a TTL cut-off of 25,000 copies/μL [18]. Piñero-Madrona et al. [19] validated the prognostic value of TTL in 5-year DFS proposed in the PLUTTO study. The NEOVATTL study demonstrated the prognostic value of TTL measured by OSNA in BC patients post-NAST, showing a clear decrease in survival at TTL ≥ 25,000 copies/μL [20]. Moreover, a prognostic scoring tool that considered TTL, Ki67 score before NAST, and treatment response in the primary tumor (Miller–Payne grade) developed in the NEOVATLL study found decreased DFS in patients with a high TTL (> 25,000 copies/μL), high Ki67 (> 20%), or low Miller–Payne grade (1 or 2).
This retrospective study aimed to validate the classification of patients in progression risk groups based on TTL values and the predictive value of TTL regarding LN affectation obtained in the NEOVATTL study in a multi-centric cohort. Additionally, we aimed to establish correlations between TTL values and the risk of positive LNs.
Materials and methods
Study design and population
This was an observational, retrospective, international, multicenter study including patients with infiltrating BC. Patients’ electronic medical records (EMR) were obtained from nine Spanish and two Italian hospitals. Patients who received NAST followed by SLNB with OSNA before December 2015 were included in the study. The SLNB was performed according to each center’s protocol, using Tc99 and/or blue dye to map the SLN. Patients under 18 years of age, with carcinoma in situ or other malignant neoplasms, those considered by the investigator to be unsuitable for the study, and those who underwent OSNA in 2012 and earlier and were included in the NEOVATTL study were excluded.
Primary data recorded in EMR were collected between June and July 2019. Data were anonymized at the center of origin in compliance with Organic Law 3/2018 of December 5 on the Protection of Personal Data and guarantee of digital rights [21]. This study was performed after obtaining approval by the Comité Ético de Investigación Clínica del Departamento de Salud de La Ribera (Clinical Research Ethics Committee of La Ribera Health Department). Furthermore, it was developed following the ethical principles originating from the latest version of the Declaration of Helsinki accepted by local authorities and which are in line with Good Clinical Practice (GCP) and the requirements of current Spanish regulations.
Endpoints and variables
The main objective of this study was to validate the classification system for prognosis, in terms of DFS, based on TTL values (according to the risk of progression TTL > 250, TTL 250−25,000, and TTL > 25,000 CK19 mRNA copies/μL), and the scoring tool (TTL + Ki67 + Miller–Payne) developed in the NEOVATTL study. As a secondary objective, this study aimed to validate the results obtained in the NEOVATTL study regarding the use of TTL values to predict non-SLN involvement.
Demographic (i.e., age), surgical, and pathologic tumor characteristics before and after NAST were collected from medical records. Surgery characteristics included data related to SLNB (TTL) and ALND (yes/no; metastatic nodes/non-sentinel nodes removed). Tumor characteristics included histological type, grade, hormonal receptors status (i.e., estrogen and progesterone receptors), and molecular subtype pre- and post-NAST. The date of the last follow-up visit and disease progression (with date) were additionally recorded.
Details on study definitions, including TTL, DFS, and prognostic scores are provided in the supplementary materials file.
Statistical analysis
A descriptive analysis of all the variables of this and the previous study (NEOVATTL) was performed by study and on pooled data from both studies. Quantitative variables were described using the mean and the standard deviation (SD) and qualitative variables were described as absolute and relative frequencies with the 95% confidence interval (95% CI). The study populations were compared using Fisher’s exact, Pearson chi-squared, and Mann–Whitney tests.
The primary validation purpose focused on applying the multivariate Cox regression model built in the NEOVATTL study to the data of a validation cohort. The variables included in the model were TTL with a cut-off point of 25,000 copies/μL, the cell proliferation index Ki67 with a cut-off point of 20%, and the Miller–Payne classification.
The framework established by Royston and Altman was followed to perform a rigorous validation [22], which initially involved the execution of a calibration test. This calibration test assesses the concordance between the survival probabilities predicted by the initial model and those observed in the validation data of this study, with adequate concordance being an indicator of successful calibration and thus providing confidence in the model's predictions.
A crucial aspect at this stage was the calculation of the Prognostic Index (PI) or calibration slope, a weighted sum of the model variables using the regression coefficients estimated by the Cox model. The PI is crucial in validation, providing a quantitative risk metric regarding calibration and discrimination in the validation data set. Additionally, we investigated whether the PI was statistically different from 1, indicating a difference in discrimination between the original and validation data sets: a PI < 1 may indicate that the validation model underestimates the risk in certain cases; contrarily, a PI > 1 may indicate that the validation model overestimates the risk. In both scenarios, a PI different from one indicates that the model's discrimination is less accurate in the validation data set compared to the original data set.
The final validation stage focused on applying the Likelihood Ratio Test (LRT) to compare the Hazard Ratios (HR) between cohorts in the models derived from the original and validation data sets. The LRT provides statistical evidence of consistency or discrepancy in the risk coefficients between the two models. Furthermore, the stratification in four risk groups using the scoring system developed in the NEOVATTL study was validated using PI calibration and the LRT comparing the HR between the two models.
For the secondary objective, areas under the receiver operating characteristic (ROC) curve (AUC) were determined to evaluate the predictive value of TTL for axillary non-SLN involvement in the validation cohort. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of TTL were evaluated at different cut-off points. The probability of finding positive ALND according to the cut-off points was evaluated by dividing false negatives by a sum of false negatives and true negatives.
The sample size was estimated at 253 patients. This estimate was based on a multivariate Cox regression that allows us to detect a change in the recurrence rate (12.4% according to the NEOVATTL study) with a hazard ratio of 2.96 for the adjusted TTL model, assuming a standard deviation for this variable of 0.5348 (calculated based on the results of the NEOVATTL study) and a moderate correlation (0.2524) with the rest of the variables included in the model. This patient number allowed conclusions to be derived with 80% statistical power and an alpha error of 5%. All analyses were performed using R software version 4.2.1.
Results
Characteristics of the study population
A total of 263 patients were included in this validation study. Additionally, data from this validation cohort and previous data from the NEOVATTL study were analyzed together, resulting in 577 patients for pooled analyses. Those patients with SLNB between 2012 and 2015 were included, and therefore, follow-up times until the data collection in 2019 might vary. The mean age of the patients in this study was 51.4 (± SD 10.5) years. Pathologic tumor characteristics pre- and post-NAST in both cohorts are described in Table S2.
TTL and surgery characteristics
Most patients had TTL values < 25,000 CK19 mRNA copies/μL with no significant differences between cohorts (Table 1). Regarding the characteristics of the surgery, summarized in Table 2, approximately 60% of patients did not undergo ALND with no significant differences between cohorts. In both cohorts, most patients had 1−2 SLN dissected for biopsy, even though a higher proportion of patients in the NEOVATTL cohort had > 2 SLN (p < 0.0001). Around 70% of patients had < 9 non-SLN removed, with no significant differences between studies. Most patients in the validation cohort lacked metastatic SLN compromise in the SLNB post-NAST, whereas most patients in the NEOVATLL cohort had 1−3 compromised SLN (p < 0.0001). The NEOVATTL Validation study included a completely different population recruited in different centers, likely explaining this difference between cohorts (Table 2).
TTL value as prognostic factor for progression
The Kaplan–Meier curves evaluating time to disease progression in three patient groups according to TTL values showed that patients with TTL > 25,000 copies/μL had a shorter DFS (Fig. 1). DFS at 5 years in patients with TTL > 25,000 copies/μL was 68.5% (95% CI 57.7−81.3%) years, compared to 90.6% (95% CI 87.4−93.8%) and 87.2% (95% CI 81.9−92.9%) years in patients with TTL < 250 and TTL 250–25,000 copies/μL, respectively. Kaplan–Meier curves were similar in both cohorts analyzed separately and jointly, confirming the value of TTL > 25,000 copies/μL as cut-off for disease prognosis (Fig. 1).
The validation cohort was stratified on risk levels according to three prognostic factors identified in the NEOVATTL: TTL, Ki67, and Miller–Payne. HR for disease progression obtained in the multivariate Cox regression model according to TTL (> 25,000 vs. ≤ 25,000 copies/μL) and Ki67 (> 20% vs. ≤ 20%) were similar and statistically significant in both cohorts, showing increased risk of progression in patients with TTL > 25,000 copies/μL (HR 3.561 and 3.665 in NEOVATTL and validation cohorts, respectively) and those with Ki67 > 20% (HR 4.131 and 2.001 in NEOVATTL and validation cohorts, respectively) (Table 3). HR values for Miller–Payne grades were lower compared to those for TTL values in both studies (HR 1.407 and 1.310 in NEOVATTL and validation cohorts, respectively) with no statistical significance in the validation cohort.
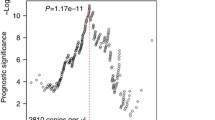
PI analysis of the multivariate Cox models for the TTL, Ki67, and Miller–Payne variables yielded a PI coefficient of 0.783 (95% CI 0.409–0.191, p < 0.0001), as shown in Figure S1. This coefficient was close to 1 (− 0.22, 95% CI − 0.591–0.157) and not significantly different from 1 (p = 0.2553), suggesting a congruence between the predictions made by the original NEOVATTL model and those observed in the validation cohort.
Additionally, the HR obtained in the NEOVATTL and validation cohorts for all prognostic factors using the LRT were compared, yielding no statistically significant differences (p = 0.226) between the two multivariate models (Table S4).
Scoring system value as prognostic factor for progression
Kaplan–Meier curves evaluating DFS according to prognostic score were similar in both cohorts analyzed separately and jointly, showing a clear difference in risk of progression in those patients with a score of 4 (Figure S2).
PI analysis of the multivariate Cox models for the prognostic score variables yielded a PI coefficient of 0.738 (95% CI 0.385−1.091; p < 0.0001), as depicted in Figure S3. This coefficient is close to 1 (− 0.26, 95% CI 0.615 – 0.091) and not significantly different from 1 (p = 0.1454), suggesting congruence between the predictions made by the original NEOVATTL model and those observed in the validation cohort. Additionally, the HR obtained in the NEOVATTL and validation cohorts for all prognostic factors using the LRT were compared, yielding no statistically significant differences (p = 0.2) between the two multivariate models (Table S4).
TTL values as predictive factor for LN involvement
The ROC curve analysis in the NEOVATTL study demonstrated concordance between TTL and non-SLN involvement, with an AUC of 0.87 (Fig. 2A). At a TTL cut-off of > 15,000 copies/μL, the sensitivity was 95.7%, the specificity 92.9%, with PPV of 51.3% and NPV of 90.5% (Table S5). ROC analysis using data from the validation cohort showed similar results regarding the concordance between TTL and non-SLN involvement, with an AUC of 0.828 (Fig. 2B). At a TTL cut-off of > 15,000 copies/μL, the sensitivity was 58.3%, the specificity 93%, with PPV of 58.3%, and NPV of 93% (Table S5). The probability of finding positive ALND according to the cut-off points was 4.54%, 7%, and 8.2% for the 250, 15,000, and 25,000 copies/μL cut-offs, respectively.
Discussion
This retrospective study of patients with infiltrating BC (who received NAST followed by SLNB with OSNA) validated the findings of the NEOVATTL study using the same TTL cut-off points to assess the risk of disease progression and DFS (< 250, 250−25,000, and TTL > 25,000 copies/μL), confirming that DFS was significantly shorter among patients with TTL > 25,000 copies/μL than patients with TTL ≤ 25,000 copies/μL. The classification system for prognosis (i.e., risk of progression) based on TTL values, and the scoring tool (TTL + Ki67 + Miller–Payne) obtained in the NEOVATTL study was also validated. A TTL value > 15,000 copies/μL also predicted non-SLN involvement in ALND.
Several histopathologic systems have been described to estimate residual disease and to stratify the prognosis in patients undergoing NAST for BC [23]. Two of the most used scores are the Miller–Payne grading system and the Residual Cancer Burden (RCB) index, which rely on histopathological analysis. Both scores demonstrated to be time-consuming and subjective [22, 23]. On the other hand, Ki67 is a cell proliferation biomarker and its expression in BC has been associated with a worse prognosis and a better response to chemotherapy [24]. The SLNB has been accepted as a good tool for establishing the axillary status in patients with and without NAST, aiming to avoid unnecessary ALND, determine prognosis, and guide treatment decisions. However, the histological assay in SLN is limited due to tissue alterations resulting from the previous systemic treatment in patients receiving NAST and the impossibility of studying the entire lymph node [11, 15]. Furthermore, histological changes post-NAST lack an impact on prognosis and staging according to the latest edition of the American Joint Committee on Cancer (AJCC) [25]. In this regard, OSNA analysis, as a molecular method, may avoid these tissue-associated limitations and provide a highly sensitive and specific quantitative result for the detection of micro- and macro-metastases of LNs compared to conventional histological examination [9, 17].
The NEOVATTL study developed a prognostic scoring tool for DFS, which included TTL as a molecular biomarker, the Ki67 score before NAST, and Miller–Payne grade. Based on this score, DFS decreased with a high TTL (≥ 25,000 copies/μL), high Ki67 (> 20%), or low Miller–Payne grade (Grade 1 or 2). In this study, these prognostic factors were also evaluated independently and combined as a prognostic scoring tool. Although the factors were overall validated, the Miller–Payne classification system showed a lower prognostic value compared to TTL values (decreased HR with no consistent statistical significance). These results suggest a superior prognostic value for TTL. Moreover, the prognostic score was validated showing a clear difference in risk of progression in those patients with a score of 4 (TTL of ≥ 25,000 copies/μL and a Ki67 of > 20%). Altogether, we further validated the proposal of Vieites et al. in the NEOVATTL study [20], regarding the value of this score for disease prognosis in BC patients after ALND.
Although ALND has been associated with postoperative complications and significant morbidity, the surgical strategy for the axillary approach, based on the results of the SLN study, continues to vary widely in clinical practice [26, 27]. This study also validated the results of the NEOVATTL study in which a cut-off of > 15,000 copies/μL TTL was found to be predictive of non-SLN involvement, reinforcing the value of this parameter. These results suggest that restricting ALND to those patients with TTL > 15,000 copies/μL is a reliable approximation for limiting overtreatment or unnecessary ALND and adding more precision to axillary surgical management.
Conventionally, prognostic risk assessment and axillary approach decisions for BC patients have been based on histopathologic methods and criteria. However, the application of these methods is limited due to their low sensitivity, especially for the detection of micro-metastases [28,29,30]. In this regard, the OSNA method has demonstrated high concordance with histopathological methods with high sensitivity, specificity, and negative predictive value. Moreover, the OSNA assay is a molecular method performed by an automated procedure, with clear advantages in terms of standardization, reproducibility, and objectivity that detects minimal tumor loads that are not always possible to detect with histological methods after NAST. The NEOVATTL study robustly demonstrated the prognostic and predictive value of TTL values assessed by OSNA assay in BC patients undergoing NAST [20]. In the era of individualized treatments, sensitive and specific tools are needed to establish disease prognosis and predict LN involvement. Our study was able to validate with a large sample of patients the results of NEOVATTL, confirming the usefulness of the OSNA method in the classification of patients in different degrees of risk of disease progression and the probability of DFS in addition to the prediction of non-SLN involvement in ALND. The limitations of this study are attributable to the retrospective nature of its design, including the risk of patient selection bias. There were also differences in patient follow-up times between studies but in both studies all patients had at least 5 years of follow-up.
Despite these limitations, the results of this study add evidence that could be relevant to developing protocols using TTL cut-off points to evaluate the risk of progression and to guide individualized surgical strategies regarding the axillary approach in BC patients who have received NAST positioning OSNA assay as a decisive tool for therapeutic de-escalation in post-neo-adjuvant treatment. These results may also increase interest in the generation of prospective, multicenter, randomized, controlled studies to determine the predictive value of TTL for the diagnosis of non-SLN metastases and to establish cut-off points that can guide more conservative surgical strategies in terms of the axillary approach after NAST in patients with BC.
Conclusion
This study externally validated the NEOVATTL model in a different multi-centric cohort. In BC patients receiving NAST and undergoing OSNA-based SLNB analysis, a TTL > 25,000 copies/μL indicated higher disease recurrence risk, while TTL > 15,000 copies/μL predicted non-SLN involvement. These findings support ALND restriction to TTL > 15,000 copies/μL as a reliable approach, minimizing unnecessary morbidity.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Li X, Wang M, Wang M, Yu X, Guo J, Sun T, et al. Predictive and prognostic roles of pathological indicators for patients with breast cancer on neoadjuvant chemotherapy. J Breast Cancer. 2019;22:497.
Popat S, Smith IE. Re: neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. JNCI J Natl Cancer Inst. 2005;97:858–858.
Kaufmann M, Von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, et al. Recommendations from an international consensus on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–16.
Mamounas EP. Optimizing surgical management of the axilla after neoadjuvant chemotherapy: an evolving story. Ann Surg Oncol. 2018;25:2124–6.
Cserni G, Maguire A, Bianchi S, Ryska A, Kovács A. Sentinel lymph node assessment in breast cancer—an update on current recommendations. Virchows Arch. 2022;480:95–107.
Bernet L, Piñero A, Martínez M, Sicart SV, Algara M, Palomares E. Consenso de la Sociedad Española de Senología y Patología Mamaria (SESPM) sobre la biopsia selectiva del ganglio centinela (BSGC) y el manejo axilar en el cáncer de mama. Revista de Senología y Patología Mamaria. 2022;35:243–59.
Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen international consensus guidelines for treatment of early breast cancer. Ann Oncol. 2021;32:1216–35.
Vasconcelos A, Melo Abreu E, Gonçalo M, Rodrigues V, André S, Schmitt F, et al. X Consenso Nacional De Cancro Da Mama - Sociedade Portuguesa de Senologia. 2022.
Navarro-Cecilia J, Dueñas-Rodríguez B, Luque-López C, Ramírez-Expósito MJ, Martínez-Ferrol J, Ruíz-Mateas A, et al. Intraoperative sentinel node biopsy by one-step nucleic acid amplification (OSNA) avoids axillary lymphadenectomy in women with breast cancer treated with neoadjuvant chemotherapy. Eur J Surg Oncol (EJSO). 2013;39:873–9.
Loibl S, von Minckwitz G, Raab G, Blohmer J-U, Costa SD, Gerber B, et al. Surgical procedures after neoadjuvant chemotherapy in operable breast cancer: results of the GEPARDUO trial. Ann Surg Oncol. 2006;13:1434–42.
Pecha V, Kolarik D, Kozevnikova R, Hovorkova K, Hrabetova P, Halaska M, et al. Sentinel lymph node biopsy in breast cancer patients treated with neoadjuvant chemotherapy. Cancer. 2011;117:4606–16.
Vieites B, López-García MÁ, Castilla C, Hernández MJ, Biscuola M, Alfaro L, et al. CK19 expression in breast tumours and lymph node metastasis after neoadjuvant therapy. Histopathology. 2016;69:239–49.
Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403–39.
Espinosa-Bravo M, Sansano I, Pérez-Hoyos S, Ramos M, Sancho M, Xercavins J, et al. Prediction of non-sentinel lymph node metastasis in early breast cancer by assessing total tumoral load in the sentinel lymph node by molecular assay. Eur J Surg Oncol (EJSO). 2013;39:766–73.
Espinosa-Bravo M, Navarro-Cecilia J, Ramos Boyero M, Diaz-Botero S, Dueñas Rodríguez B, Luque López C, et al. Intraoperative assessment of sentinel lymph node by one-step nucleic acid amplification in breast cancer patients after neoadjuvant treatment reduces the need for a second surgery for axillary lymph node dissection. Breast. 2017;31:40–5.
Takamoto K, Shimazu K, Naoi Y, Shimomura A, Shimoda M, Kagara N, et al. One-step nucleic acid amplification assay for detection of axillary lymph node metastases in breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2016;23:78–86.
Peg V, Espinosa-Bravo M, Vieites B, Vilardell F, Antúnez JR, de Salas MS, et al. Intraoperative molecular analysis of total tumor load in sentinel lymph node: a new predictor of axillary status in early breast cancer patients. Breast Cancer Res Treat. 2013;139:87–93.
Peg V, Sansano I, Vieites B, Bernet L, Cano R, Córdoba A, et al. Role of total tumour load of sentinel lymph node on survival in early breast cancer patients. Breast. 2017;33:8–13.
Piñero-Madrona A, Ripoll-Orts F, Sánchez-Méndez JI, Chaves-Benito A, Gómez-de la Bárcena MR, Calatrava-Fons A, et al. External validation of a prognostic model based on total tumor load of sentinel lymph node for early breast cancer patients. Breast Cancer Res Treat. 2020;181:339–45.
Vieites B, López-García MÁ, Martín-Salvago MD, Ramirez-Tortosa CL, Rezola R, Sancho M, et al. Predictive and prognostic value of total tumor load in sentinel lymph nodes in breast cancer patients after neoadjuvant treatment using one-step nucleic acid amplification: the NEOVATTL study. Clin Transl Oncol. 2021;23:1377–85.
Boletín Oficial del Estado. Ley Orgánica 3/2018, de 5 de diciembre, de Protección de Datos Personales y garantía de los derechos digitales. [Internet]. 2018. [cited 2022 Nov 29]. Available from: https://www.boe.es/eli/es/lo/2018/12/05/3/con
Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol [Internet]. 2013;13:1–15.
Wang W, Liu Y, Zhang H, Zhang S, Duan X, Ye J, et al. Prognostic value of residual cancer burden and Miller–Payne system after neoadjuvant chemotherapy for breast cancer. Gland Surg. 2021;10:3211–21.
Panal Cusati M, Herrera de la Muela M, Hardisson Hernaez D, Choqueneira Dionisio M, Román Guindo A, de Santiago Garcia FJ. Correlación entre la expresión de Ki67 con factores clásicos pronósticos y predictivos en el cáncer de mama precoz. Revista de Senología y Patología Mamaria. 2014;27:163–9.
Amin MB, Frederick GL, Edge SB, Compton CC, Gershenwald JE, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin [Internet]. 2017;67:93–9.
Invernizzi M, Michelotti A, Noale M, Lopez G, Runza L, Giroda M, et al. Breast cancer systemic treatments and upper limb lymphedema: a risk-assessment platform encompassing tumor-specific pathological features reveals the potential role of trastuzumab. J Clin Med. 2019;8:138.
Jud SM, Hatko R, Maihöfner C, Bani MR, Schrauder MG, Lux MP, et al. Comprehensive visualization of paresthesia in breast cancer survivors. Arch Gynecol Obstet. 2014;290:135–41.
van de Vrande S, Meijer J, Rijnders A, Klinkenbijl JHG. The value of intraoperative frozen section examination of sentinel lymph nodes in breast cancer. Eur J Surg Oncol (EJSO). 2009;35:276–80.
Tew K, Irwig L, Matthews A, Crowe P, Macaskill P. Meta-analysis of sentinel node imprint cytology in breast cancer. Br J Surg. 2005;92:1068–80.
Motomura K, Inaji H, Komoike Y, Kasugai T, Nagumo S, Noguchi S, et al. Intraoperative sentinel lymph node examination by imprint cytology and frozen sectioning during breast surgery. Br J Surg. 2002;87:597–601.
Acknowledgements
The authors thank the i2e3 Procomms team and specially, Sara Gutiérrez, MD and Sara Cervantes, PhD, for providing medical writing support during the preparation of the manuscript.
Funding
This work was supported by Sysmex in the medical writing of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by LB-V and all authors revised previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bernet-Vegué, L., Cantero-González, C., Sancho de Salas, M. et al. Validation of prognostic and predictive value of total tumoral load after primary systemic therapy in breast cancer using OSNA assay. Clin Transl Oncol 26, 1220–1228 (2024). https://doi.org/10.1007/s12094-023-03347-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03347-7