Abstract
Purpose
Immune checkpoint inhibitors (ICIs) have been incorporated in the treatment of metastatic urothelial carcinoma (mUC) upon platinum-based chemotherapy according to the positive results of large clinical trials. Nevertheless, results from unselected populations reflecting real-world data (RWD) are highly informative to the clinician. We reviewed daily clinical practice outcomes in patients with mUC who received atezolizumab in our institution.
Methods
Here we evaluated the clinical activity and safety of atezolizumab in an unselected population of mUC patients who received atezolizumab between 2018 and 2022 reflecting RWD. Efficacy and safety information were retrospectively collected.
Results
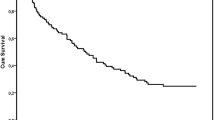
A total of 63 patients were included. The mean age was 68 years and the objective response rate was 14.3%. The median progression-free survival was 3 months and the median overall survival 6 months. At 1 year, 42% of the patients were alive. ECOG (0 vs 1) and neutrophil–lymphocytes ratio < 2 at the start of ICI were positive prognostic factors that discriminated between long vs short survivors. Overall tolerance was good with no new safety signals. Five patients (17%) had treatment-related adverse events grade ≥ 2 that required corticosteroids.
Conclusion
In this retrospective study, atezolizumab was an effective and tolerable treatment option for patients with mUC after progression to platinum-based chemotherapy. Yet, patient selection remains critical to improve outcomes.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author, [ID].
References
WHO Cancer Mortality Databse. In: https://gco.iarc.fr/today/data/factsheets/cancers/30-Bladder-fact-sheet.pdf. Accesed 26 Feb 2023
Bedke J, Albiges L, Capitanio U, Giles RH, Hora M, Ljunberg B, et al. The 2022 updated European association of urology guidelines on the use of adjuvant immune checkpoint inhibitor therapy for renal cell carcinoma. Eur Urol. 2023;83(1):10–4. https://doi.org/10.1016/j.eururo.2022.10.010.
Valderrama BP, González-Del-Alba A, Morales-Barrera R, Peláez Fernández I, Vázquez S, Caballero Díaz C, et al. SEOM-SOGUG clinical guideline for localized muscle invasive and advanced bladder cancer (2021). Clin Transl Oncol. 2022;24(4):613–24. https://doi.org/10.1007/s12094-022-02815-w.
Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011;306(7):737–45. https://doi.org/10.1001/jama.2011.1142.
Liu S, Yang T, Na R, Hu M, Zhang FuY, et al. The impact of female gender on bladder cancer-specific death risk after radical cystectomy: a meta-analysis of 27912 patients. Int Urol Nephrol. 2015;47(6):951–8. https://doi.org/10.1007/s11255-015-0980-6.
Shi ZD, Hao L, Han XX, Wu ZX, Pang K, Dong Y, et al. Targeting HNRNPU to overcome cisplatin resistance in bladder cancer. Mol Cancer. 2022;21(1):37. https://doi.org/10.1186/s12943-022-01517-9.
Mollica V, Rizzo A, Montironi R, Cheng L, Giunchi F, Schiavina R, et al. Current strategies and novel therapeutic approaches for metastatic urothelial carcinoma. Cancers (Basel). 2020;12(6):1449. https://doi.org/10.3390/cancers12061449.
Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmström PU, Choi W, et al. Bladder cancer. Lancet. 2016;388(10061):2796–810. https://doi.org/10.1016/S0140-6736(16)30512-8.
Chierigo F, Wenzel M, Würnschimmel C, Flammia RS, Horlemann B, Tian Z, et al. Immuno-oncology therapy in metastatic bladder cancer: A systematic review and network meta-analysis. Crit Rev Oncol Hematol. 2022. https://doi.org/10.1016/j.critrevonc.2021.103534.
Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748–57. https://doi.org/10.1016/S0140-6736(17)33297-X.
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. KEYNOTE-045 investigators pembrolizumab as second-line therapy for advanced urothelial Carcinoma. N Engl J Med. 2017. https://doi.org/10.1056/NEJMoa1613683.
Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–22. https://doi.org/10.1016/S1470-2045(17)30065-7.
Balar AV, Castellano DE, Grivas P, Vaughn DJ, Powles T, Vuky J, et al. Efficacy and safety of pembrolizumab in metastatic urothelial carcinoma: results from KEYNOTE-045 and KEYNOTE-052 after up to 5 years of follow-up. Ann Oncol. 2023;34(3):289–99. https://doi.org/10.1016/j.annonc.2022.11.012.
Van der Heijden MS, Loriot Y, Durán I, Ravaud A, Retz M, Vogelzang NJ, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma: a long-term overall survival and safety update from the phase 3 IMvigor211 clinical trial. Eur Urol. 2021;80(1):7–11. https://doi.org/10.1016/j.eururo.2021.03.024.
Sternberg CN, Loriot Y, James N, Choy E, Castellano D, Lopez-Rios F, et al. Primary results from SAUL, a multinational single-arm safety study of Atezolizumab therapy for locally advanced or metastatic urothelial or nonurothelial carcinoma of the urinary tract. Eur Urol. 2019;76(1):73–81. https://doi.org/10.1016/j.eururo.2019.03.015.
Powles T, Park SH, Voog E, Caserta C, Valderrama B, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic Urothelial Carcinoma. N Engl J Med. 2020;383(13):1218–30. https://doi.org/10.1056/NEJMoa2002788.
Bamias A, Merseburger AS, Loriot Y, James N, Choy E, Castellano D, et al. SAUL, a single-arm study of atezolizumab for chemotherapy-pretreated locally advanced or metastatic carcinoma of the urinary tract: outcomes by key baseline factors, PD-L1 expression and prior platinum therapy. ESMO Open. 2021. https://doi.org/10.1016/j.esmoop.2021.100152.
Sonpavde G, Manitz J, Gao C, Tayama D, Kaiser C, Hennessy D, et al. Five-factor prognostic model for survival of post-platinum patients with metastatic urothelial carcinoma receiving PD-L1 inhibitors. J Urol. 2020;204(6):1173–9. https://doi.org/10.1097/JU.0000000000001199.
Galsky M, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, et al. PD10–01 disease-free survival with longer follow-up from the checkmate 274 trial of adjuvant Nivolumab in patients after surgery for high-risk muscle-invasive urothelial carcinoma. J Urology. 2022. https://doi.org/10.1097/JU.0000000000002536.01.
Khaki AR, Li A, Diamantopoulos LN, Bilen MA, Santos V, Esther J, et al. Impact of performance status on treatment outcomes: a real-world study of advanced urothelial cancer treated with immune checkpoint inhibitors. Cancer. 2020;126(6):1208–16. https://doi.org/10.1002/cncr.32645.
Swami U, Haaland B, Kessel A, Nussenzveig R, Maughan BL, Esther J, et al. Comparative effectiveness of immune checkpoint inhibitors in patients with platinum refractory advanced urothelial carcinoma. J Urol. 2021;205(3):709–17. https://doi.org/10.1097/JU.0000000000001412.
Sotelo M, Alonso-Gordoa T, Gajate P, Gallardo E, Morales-Barrera R, Pérez-Gracia JL, et al. Atezolizumab in locally advanced or metastatic urothelial cancer: a pooled analysis from the Spanish patients of the IMvigor 210 cohort 2 and 211 studies. Clin Transl Oncol. 2021;23(4):882–91. https://doi.org/10.1007/s12094-020-02482-9.
Pond GR, Agarwal A, Ornstein M, Garcia J, Gupta R, Grivas P, et al. Clinical outcomes of platinum-ineligible patients with advanced urothelial carcinoma treated with first-line PD1/L1 inhibitors. Clin Genitourin Cancer. 2021;19(5):425–33. https://doi.org/10.1016/j.clgc.2021.04.008.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MS, NMU, and AM. The first draft of the manuscript was written by MS, ID, and NMU and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors did not receive support from any organization for the submitted work and have no relevant or nonfinancial interests to disclose.
Ethical approval
The study protocol was approved by the Clinical Research Ethics Committee of Cantabria.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sotelo, M., Muñoz-Unceta, N., Matorras, A. et al. Outcomes with atezolizumab in metastatic urothelial cancer: real-world data from a single institution. Clin Transl Oncol 26, 682–688 (2024). https://doi.org/10.1007/s12094-023-03288-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03288-1