Abstract
Colorectal cancer (CRC) is the second leading cause of cancer deaths in Spain. Metastatic disease is present in 15–30% of patients at diagnosis and up to 20–50% of those with initially localized disease eventually develop metastases. Recent scientific knowledge acknowledges that this is a clinically and biologically heterogeneous disease. As treatment options increase, prognosis for individuals with metastatic disease has steadily improved over recent decades. Disease management should be discussed among experienced, multidisciplinary teams to select the most appropriate systemic treatment (chemotherapy and targeted agents) and to integrate surgical or ablative procedures, when indicated. Clinical presentation, tumor sidedness, molecular profile, disease extension, comorbidities, and patient preferences are key factors when designing a customized treatment plan. These guidelines seek to provide succinct recommendations for managing metastatic CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Incidence and epidemiology
Colorectal cancer (CRC) has the third highest incidence of all cancers worldwide, with 2022 estimates of 1,931,590 cases (10.7%), and the second highest mortality, with 935,173 deaths (9.4%) in 2020 [1]. It has been estimated that it will be the most frequently diagnosed tumor in Spain, with 43,370 new cases in 2022. In 2020, the estimated prevalence at 5 years was 191,884 cases. CRC was responsible for 11,131 deaths in 2020, with an expected increase to 17,735 by 2040 [2]. Approximately 20% of patients with CRC have metastases at the time of diagnosis, while up to 50% of those whose disease was initially localized will develop metastases. More often than not, metastatic disease is non-curable and median survival times do not exceed 30 months.
Most cases are sporadic (75–80%) and approximately 20% present familial aggregation. Only 5–7% cases are due to germline deleterious genetic variants that cause known hereditary diseases, such as Lynch syndrome and familial adenomatous polyposis [3]. Of the risk factors for sporadic CRC, aging is the most important one and has been growing exponentially through the years. Other related factors include inflammatory bowel disease and environmental factors, some of which are modifiable, such as obesity, sedentary lifestyle, diet rich in red and/or processed meats and low in fiber, and alcohol and tobacco use [4].
Methodology
This guideline is based on a systematic review of relevant published studies and with the consensus of ten oncologist experts in treatment from two Spanish digestive cooperative groups (Grupo Español Multidisciplinar de Cáncer Digestivo, GEMCAD, and Grupo Español de Tumores Digestivos, TTD), the Spanish Society of Medical Oncology (SEOM), and an external review panel comprising two experts designated by SEOM. The Infectious Diseases Society of America–US Public Health Service Grading System for Ranking Recommendations in Clinical Guidelines has been used to assign levels of evidence and grades of recommendation [5] (Table 1).
Diagnosis and staging
Upon suspicion of CRC based on suggestive symptoms or screening tests, a complete colonoscopy with biopsy to locate the primary tumor and confirm the pathological diagnosis is mandatory. Virtual colonoscopy is an alternative to detect potential synchronous colorectal lesions if a full colonoscopy is not feasible [I, A] [6, 7].
Diagnostic procedures should include a complete medical history (comorbidities, previous oncologic treatments, and family history of cancer), symptoms related to disease, as well as performance status, clinical examination, and laboratory tests (liver and renal function, blood count, and serum carcinoembryonic antigen) [8].
Computed tomography (CT) scan of the chest, abdomen, and pelvis is the best technique to assess distant extent [IV, A]. Magnetic resonance imaging (MRI) of the liver might be considered in certain cases, such as resectable or potentially resectable hepatic metastases [IV, A], evaluation of locally advanced tumors (especially rectal cancer), or for patients with iodine-contrast allergies, chronic kidney disease, or hepatic steatosis. A 18 F-fluorodeoxyglucose positron emission tomography (PET–CT) scan is not routinely recommended, but should be contemplated in selected subjects with increased tumor markers without evidence of metastatic disease or those with oligo-metastatic diseases that are potentially curable with local treatments [IV, B] [9].
To optimize the treatment strategy, individuals with metastatic colorectal cancer (mCRC) should be evaluated by an experienced multidisciplinary team at diagnosis and later, if necessary [III, A] [10]. The recommended staging system is that of the 8th edition of the American Join Committee on Cancer (AJCC) [I, A] [11].
Recommendations
-
A complete colonoscopy with biopsy to confirm the diagnosis is mandatory. Virtual colonoscopy is an alternative to detect potential synchronous colorectal lesions if a full colonoscopy is not feasible [I, A].
-
CT scan of the chest, abdomen, and pelvis is the best technique to assess distant metastases [IV, A].
-
MRI and PET-CT may be considered in selected cases [IV, B].
-
Patients with mCRC should be evaluated by a multidisciplinary team to define patient management: resectable, potentially resectable, and un-resectable disease [III, A].
-
The recommended staging system is that of the 8th edition of the AJCC [I, A].
Biomarkers
Molecular profiling of mCRC and identification of specific biomarkers and molecular targets can serve as predictive and prognostic indicators of disease and response to targeted therapies. When incorporated into clinical decision-making, biomarkers and molecular targets are critical tools in personalizing therapy.
Testing for RAS (KRAS/NRAS) and BRAF mutations, as well as high microsatellite instability (MSI-H) or deficient mismatch repair proteins (dMMR) is recommended in all cases at the time of mCRC diagnosis [I, A] [12,13,14]. Liquid biopsy can be contemplated for molecular profiling when conventional tumor biopsy is not available or to monitor emergent mutations of resistance to targeted therapy, especially prior to re-challenge with anti-epidermal growth factor receptor (anti-EGFR) treatment, although this has yet to be approved by our national authorities [II, B] [15].
In pivotal randomized clinical trials in mCRC, the role of KRAS and NRAS (exons 2, 3, and 4) hotspot mutations has been demonstrated as negative predictive factors for response to anti-EGFR monoclonal antibodies (MoAbs) [12, 16,17,18,19]. Beyond these widely approved biomarkers, primary tumor location has been reported to be predictive of response to these therapies in the front-line setting of all RAS/BRAF wild-type tumors. In two pooled analyses including six randomized clinical trials, participants with left-sided tumors exhibited better overall survival, progression-free survival, and response rate to first-line chemotherapy combinations plus anti-EGFR agents, whereas individuals with right-sided tumors benefited more from standard chemotherapy + / − bevacizumab [20,21,22].
BRAF V600E mutations have proven to confer poor prognosis in advanced disease, hence the recommendation to detect their presence/absence together with RAS mutations. Moreover, the BRAF V600E mutation comprises a positive predictive factor for response to dual BRAF/EGFR inhibition in second and third line (encorafenib/cetuximab) [23].
MSI-H or dMMR must be assessed by PCR and/or immunohistochemistry (IHC) (MLH1, MSH2, MSH6, and PMS2), respectively [16], to assist clinicians with genetic counseling, including identifying Lynch syndrome [II, B], and selecting patients for immune checkpoint inhibition (ICI) [I, A] [13, 14].
HER2 overexpression by IHC or amplification by fluorescence in situ hybridization (FISH) has yet to prove its role as a poor prognosis biomarker. Several dual blockade HER2-targeted therapies have exhibited significant efficacy, although they have yet to be approved by our national authorities. Consequently, knowledge about this (covered by a prescreening research program in subsequent lines of treatment and, particularly, in RAS/BRAF wild-type populations) can contribute to determining eligibility to participate in clinical trials of these compounds [III, C] [24].
NTRK fusions constitute an uncommon molecular event in CRC, confined to RAS and BRAF wild-type tumors, predominantly in MSI-H/dMMR. This subset of patients would be eligible for undergoing the test in subsequent lines of treatment to consider accessibility to clinical trials [III, A] [25].
Regulatory agencies recommend di-hydro-pyridine dehydrogenase (DPYD) genotyping or phenotyping [III, A]. DPYD gene variants can lead to severe toxicities with fluoro-pyrimidines. Individuals harboring these alterations should receive lower doses of these compounds or even skip them for alternative regimens [26]
Next-generation sequencing (NGS) platforms have not been universally established in our country for mCRC molecular studies and are useful tools to analyze RAS, BRAF, and HER2 alterations simultaneously and providing information regarding tumor hyper-mutation burden (TMB), as well as complementary diagnostic information about Lynch syndrome by mutational study of the MMR and EPCAM genes [27].
Table 2 illustrates the staging procedures and standard biomarkers suggested for all cases of mCRC.
Recommendations
-
KRAS, NRAS exons 2, 3, and 4, and BRAF V600E mutations should be tested at the time of mCRC diagnosis [I, A].
-
Assessment of mismatch repair deficiency (IHC or MSI-H) is recommended to assist genetic counseling for Lynch syndrome [II, B] and for its predictive value of benefit from ICI [I, A].
-
Identification of HER 2 amplification or overexpression [III, C] and NTRK fusions are recommended in subsequent lines for access to clinical trials with targeted therapies [III, A].
-
Liquid biopsy might be considered to monitor emergent mutations of resistance to targeted therapy, especially prior to re-challenge with anti-epidermal growth factor receptor (anti-EGFR) treatment, though this is not supported by our national authorities [II, B].
-
Testing for DPYD deficiency is strongly recommended prior to initiating 5-fluorouracil-based chemotherapy [III, A].
Resectable disease
At diagnosis, mCRC may be technically resectable in a small percentage of patients. It is considered a potentially curable disease, with reported 5-year overall survival (OS) rates of 20–45% in a highly selected population [28]. Commonly involved sites are liver, lung, peritoneum, lymph nodes, and ovary. Resection should not be undertaken unless complete removal of all known tumor and metastatic sites (R0 resection) is feasible, because incomplete resection or de-bulking has failed to prove to prolong OS.
Resectable CRC liver metastases are defined as metastatic disease amenable to R0 resection while leaving at least 20–25% of total liver volume as future organ remnant, with adequate vascular inflow and outflow, and sufficient biliary drainage [28]. Certain preoperative factors are independent predictors of poor survival: T4 primary tumor, synchronous metastatic presentation, ≥ 4 liver metastases, diameter ≥ 5 cm in the largest liver metastasis, and serum CEA level ≥ 5 ng/ml. Based on the afore-mentioned factors, resectable cases can be divided into high risk (≥ 3 factors) and low risk (fewer than 3 factors) [10]. Other poor prognostic factors are the presence of extrahepatic metastases, > 3 cm of the primary tumor diameter, and disease-free interval from diagnosis of localized to metastatic disease < 12 months [29, 30].
Extrahepatic disease is not regarded as an absolute contraindication to surgery and resection may lead to significant OS benefit in well-selected patients [31, 32]. Ablative techniques, such as stereotactic radiotherapy (SBRT) and thermal ablation, may be contemplated alone or in conjunction with resection [10].
In subjects with resectable disease and favorable prognostic criteria, perioperative treatment may not be necessary and upfront R0 resection is recommended [III, B]. In contrast, when prognosis is unclear or unfavorable, perioperative combination chemotherapy should be pondered over [II, B] [33, 34]. The preferred treatment should be FOLFOX (or alternatively CAPOX) [II, B] [33]. EGFR-targeting antibodies (MoAbs) are not recommended in this setting [IV, B] [35]. No data with bevacizumab are available for this specific group. Adjuvant chemotherapy is not recommended for individuals with favorable oncological and technical (surgical) criteria who have not received perioperative chemotherapy [II, C], but may be beneficial for those with unfavorable criteria [IV, D] [36]. Decision-making should therefore include patient characteristics and preferences. The most recent randomized clinical phase II/III trial demonstrated that postoperative chemotherapy with mFOLFOX6 improved disease-free survival (DFS), yet failed to impact OS [37].
The treatment recommendations above may also apply to pulmonary mCRC, albeit with less evidence. Complete resection is required, while maintaining adequate function. Ablative techniques may also be considered alone or in conjunction with resection. Surgery of pulmonary metastases has achieved a 3-year recurrence-free survival in 28% and 3-year OS in 78% of the cases [38].
In subjects with limited peritoneal carcinomatosis, complete cyto-reductive surgery may provide prolonged survival when performed in high-volume centers [II, A] [39]. However, the addition of hyperthermic intraperitoneal chemotherapy (HIPEC) has not proven beneficial in randomized trials, and, therefore, cannot be recommended as a standard of care [IV, B] [40].
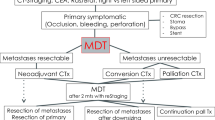
Fig. 1 illustrates the suggested treatment algorithm for individuals with mCRC limited to the liver.
Recommendations
-
Upfront resection is recommended for resectable hepatic or lung metastases with favorable prognostic criteria [III, B].
-
In resectable metastases with unfavorable prognostic factors, perioperative/adjuvant therapy with FOLFOX/CAPOX should be considered [II, B].
-
Complete cyto-reductive surgery should be performed in experienced centers [II, A]. However, the addition of HIPEC is not recommended [IV, B].
Potentially resectable disease
Conversion chemotherapy refers to patients initially diagnosed with un-resectable disease, in whom an optimal response to chemotherapy would enable resection of metastatic sites. In the case of liver resection, salvage surgery is estimated to be feasible in 12.5% of these patients following chemotherapy with or without targeted agents [41]. Potentially resectable cases are defined as those who present the following situations:
-
Post-resection liver remanent (LR) is inadequate either in volume or quality. At least 25% is the minimum safe LR volume needed after hepatic resection in individuals with a normal liver, and 40% if sinusoidal obstruction syndrome, cholestasic, steatosic, or cirrhotic liver is present. Treatment focuses on increasing LR volume and enhancing function, and strategies such as percutaneous transhepatic portal vein embolization should be discussed [42].
-
A large tumor burden compromising R0 resection.
Such cases must be down-staged to obtain negative margin resection and neoadjuvant systemic treatment and/or locoregional therapies may be needed. The objective of systemic treatment is to decrease tumor size, prevent disease progression, and eradicate any remnant of disease. Treatment response must be closely monitored every 2 months so that resection can be undertaken as soon as the metastases become resectable, avoiding unnecessary liver toxicity [III, A]. RECIST criterion, based on the metastatic size, is the standard method to evaluate treatment response. However, if treatment with anti-vascular endothelial growth factor (VEGF) MoAbs are administered, morphological response criteria for CT imaging are preferred [43].
Chemotherapy schedules are recommended with high response rates (RR) or with a short time to response. Furthermore, adding targeted agents to a cytotoxic doublet or triplet should be contemplated according to tumor side and molecular profile.
In a systematic review of patients with liver only disease treated with chemotherapy and a targeted agents, R0 resection rates of 27–52% and median OS of 20–49 months were reported. Triplet therapy demonstrated benefit in RR and R0 resection rates compared to doublets, and the addition of bevacizumab to a triplet regimen displayed higher RR and prolonged progression-free survival (PFS) [41]. RAS mutational status was found to be the most important predictive feature for conversion chemotherapy. In RAS wild-type patients, the addition of anti-EGFR agents to a cytotoxic doublet was associated with significantly higher RR, R0 resection rates, and OS [44].
Data from a retrospective pooled analysis of six trials found that patients with left-sided RAS wild-type tumors receiving chemotherapy plus EGFR MoAb therapy had higher RR, PFS, and OS than patients treated with chemotherapy alone or with chemotherapy plus bevacizumab; consequently, this therapy is recommended [II, A]. In subjects with RAS wild-type right-sided tumors, treatment with chemotherapy and anti-EGFR MoAb also exhibited higher RR, as did triplet therapy with bevacizumab. Both combinations are therefore recommended [20, 45] [II, A].
In cases of RAS mutant, un-resectable CRC liver-limited metastases, bevacizumab combined with cytotoxic doublets or triplets increased the resectability rate and improved RR and OS compared chemotherapy alone [45].
In the setting of un-resectable mCRC, transarterial radioembolization (TARE) with microspheres impregnated with yttrium-90 (Y90) together with chemotherapy was associated with a statistically significant increase in the potentially curative resectability of the liver [IV, D] [46]. Hepatic arterial infusion chemotherapy has emerged as a complementary method to systemic chemotherapy in conversion strategy [III, B] [47]. Oncosurgical strategies, such as two-stage hepatectomy (TSH) [48], a combination of surgical resection with local ablation, or ALPPS procedure (associating liver partition and portal vein ligation for stage hepatectomy) [49] may help un-resectable metastases become resectables.
Recommendations
-
A regimen leading to high RR and/or making a large tumor smaller is recommended for individuals with potentially resectable CRC liver metastases [II, A].
-
Response to treatment must be closely monitored every 2 months [III, A].
-
A cytotoxic doublet plus an anti-EGFR is recommended in cases of RAS wild-type disease and left-sided tumors [II, A].
-
For patients with RAS wild-type disease and right-sided tumors, a cytotoxic doublet plus an anti-EGFR antibody or a cytotoxic doublet or triplet plus or minus bevacizumab is recommended [II, A].
-
For subjects with RAS mutant disease, a cytotoxic doublet or triplet plus or minus bevacizumab is recommended [II, A].
Unresectable disease: First-line therapy
The cornerstone of first-line therapy for un-resectable metastases from CRC is systemic treatment. Surgery of primary tumor should only be considered in symptomatic patients. There is no evidence of increased OS as a result of resecting an asymptomatic primary tumor in cases of synchronous un-resectable metastases [II, B] [50].
The foremost treatment endpoints in this setting include prolonging survival, relieving symptoms secondary to the disease, and improving and maintaining quality of life. The most relevant factors that bear upon treatment selection (in all lines of therapy) are related to tumor (molecular profile, tumor burden, related symptoms, primary tumor sidedness, and progression dynamics), patient (performance status, comorbidities, age, and expectations), and treatment characteristics (toxicity profile, cost, and availability) [16]. In the first-line setting, delivering chemotherapy in combination with biological therapy (anti-VEGF) or anti-EGFR MoAb) is generally recommended.
Based on their general condition, patients are classified as fit or unfit. Unfit individuals must be defined by patient-related characteristics and not by tumor-derived symptoms. Clinical guidelines recommend some type of therapy in these cases (not eligible for intensive therapy [51] or potentially suitable to receive treatment) [16]. For unfit subjects with wild-type RAS/BRAF and left-sided tumors, monotherapy with anti-EGFR MoAb [IV, C] [52] or, preferably a combination of 5-fluorouracil with anti-EGFR [I, A] or 5-fluorouracil ± bevacizumab [I, B] [53, 54] are well tolerated and have displayed efficacy, whereas in unfit patients with RAS/BRAF mutation or right-sided tumors, fluoropyrimidine ± bevacizumab is the most appropriate combination [I, B] [54]. Similarly, monotherapy with fluoropyrimidine can be contemplated in individuals with certain comorbidities.
For MSI-H/dMMR tumors, ICI is the treatment of choice. Pembrolizumab has been shown to increase RR and PFS compared to chemotherapy combinations. Moreover, there was less severe toxicity with ICI. No differences in OS could be demonstrated, inasmuch as 60% of the individuals who received chemotherapy were crossed over to pembrolizumab [I, A] [13].
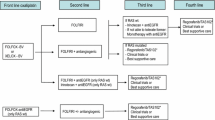
For fit subjects with microsatellite stable disease, first-line treatment consists of a chemotherapy doublet consisting of the anti-VEGF MoAbs bevacizumab or anti-EGFR agents, such as cetuximab or panitumumab (Fig. 2). Chemotherapy combinations include doublets of fluoropyrimidine (5-fluorouracil or capecitabine) plus oxaliplatin or irinotecan (FOLFOX, FOLFIRI, CAPOX). The treatment recommendation for wild-type RAS and BRAF tumors is based on primary tumor location. RR and OS are better for people with left-sided tumors who receive a combination of chemotherapy with anti-EGFR MoAb [I, A]. In contrast, for patients with right-sided tumors, a greater OS and PFS benefit has been suggested for chemotherapy alone or combined with bevacizumab [II, B] [21, 55, 56]. For RAS or BRAF-mutated tumors, the first-line treatment recommendation is chemotherapy combination (doublet or triplet) with bevacizumab [II, B]. In one meta-analysis, the chemotherapy triplet of 5-fluorouracil, oxaliplatin, and irinotecan together with bevacizumab has exhibited improved OS, PFS, and RR, with respect to doublets + bevacizumab, mainly in RAS-mutated and right-sided tumors, with a moderate increase in toxicity. The benefit of this combination is limited to selected patients (ECOG 0–1, < 70–75 years old, with no comorbidities and no previous oxaliplatin-based adjuvant chemotherapy) [I, B] [57]. For patients with wild-type RAS/BRAF, triplet in combination with an anti-EGFR mAb has proven no benefit in terms of treatment activity and increased gastrointestinal toxicity and, therefore, cannot be recommended [II, E] [58].
Recommendations concerning the duration of first-line therapy in mCRC have evolved in recent years. Historically, the disease was treated continuously until progression, unacceptable toxicity, or the patient’s wish. Currently, treatment duration is subjected to the person’s personal circumstances, cumulative treatment toxicity, response to first-line treatment, and the aggressiveness of the disease. Response should be assessed every 8–12 weeks, with treatment de-escalation allowed after 4–6 months (mainly in those patients receiving oxaliplatin) to prevent the appearance of irreversible sensory neurotoxicity. Hence, different strategies have emerged including stop-and-go or intermittent therapy, as well as maintenance treatment. After induction chemotherapy combined with bevacizumab, fluoropyrimidine maintenance with or without bevacizumab significantly improves PFS and reveals a trend toward prolonged OS and, hence, should be considered [I, B] [59]. Randomized phase II studies have shown an increase in efficacy with 5-fluorouracil with anti-EGFR MoAb maintenance versus anti-EGFR or 5-fluorouracil in monotherapy (II, B) [60].
Recommendations
-
Resection of an asymptomatic primary tumor is not recommended [II, B].
-
For unfit patients with wild-type RAS/BRAF and left-sided tumors, monotherapy with anti-EGFR agents [IV, C] or, preferably a combination of 5-fluorouracil with anti-EGFR [I, A] is recommended.
-
For unfit subjects with mutated RAS/BRAF and/or right-sided tumors, fluoropyrimidine ± bevacizumab is the most suitable combination [II, B].
-
In cases of MSI-H/dMMR tumors, ICI (pembrolizumab) is the treatment of choice [I, A].
-
A combination of chemotherapy doublet with anti-EGFR MoAb is recommended for wild-type RAS and BRAF and left-sided tumors [I, A]. Triplets plus anti-EGFR are not deemed appropriate in this context [II, E].
-
For patients with mutated RAS or BRAF and/or right-sided tumors, chemotherapy doublets with or without bevacizumab are recommended [II, B]. Triplets with bevacizumab should be considered in selected cases (ECOG 0–1, < 70–75 years old and no previous oxaliplatin-based adjuvant chemotherapy) [I, B].
-
After induction chemotherapy with bevacizumab, fluoropyrimidine with or without bevacizumab maintenance therapy should be considered [I, B].
-
After induction treatment with chemotherapy plus anti-EFR MoAbs, maintenance with fluropyrimidine plus anti-EGFR may also be contemplated [II, B]
Unresectable disease: Second-line
Almost all individuals treated with first-line therapy who do not benefit from curative treatment strategies will progress and more than 60% of them can be considered for second-line treatment. Patients should receive all available treatments during the course of the disease. Given the palliative nature of this therapy and its modest efficacy and toxicity profile, physician–patient shared decision-making is recommended. Selected cases of indolent disease and comorbidities can be managed, at least temporally, without oncological treatment.
Second-line strategy will depend on prior treatment and patient preferences. For subjects who received first-line oxaliplatin-based therapy, FOLFIRI and irinotecan alone are the preferred options. When the previous treatment was an irinotecan-based combination, the recommended options are FOLFOX and CAPOX. Recommendations on targeted therapies are based on RAS and BRAF status (Fig. 3).
For RAS-mutated tumors, bevacizumab and aflibercept added to chemotherapy are both options in second-line therapy [I, A] [61, 62]. In cases previously treated with first-line bevacizumab-containing chemotherapy, the continuation of bevacizumab in conjunction with a second-line chemotherapy improves OS compared to simply switching the chemotherapy regimen alone [63]. In the BRAF-mutated setting, the combination of encorafenib and cetuximab has exhibited increased efficacy in terms of RR, PFS, and OS with respect to cetuximab–irinotecan [I, A] [23]. Efforts should be dedicated to identifying biomarkers that could improve efficacy in this devastating subset of tumors. In double wild-type patients (RAS and BRAF WT), the standard therapy is to change the doublet and, in those not previously treated with anti-EGFR, MoAbs should be contemplated [II, C] [64, 65]. In the context of treatment with first-line chemotherapy and anti-EGFR MoAbs, the addition of bevacizumab or aflibercept in second-line is not born out of phase III clinical trials. In dMMR/MSI-H tumors progressing after first-line chemotherapy, ipilimumab plus nivolumab or pembrolizumab is recommended [66] [III, B].
Recommendations
-
For RAS-mutated neoplasms, bevacizumab and aflibercept added to chemotherapy are options in second-line therapy [I, A].
-
In BRAF-mutated tumors, the combination of encorafenib and cetuximab is recommended. However, these treatments are not yet approved by the Spanish healthcare authorities. [I, A].
-
The standard second-line therapy for RAS and BRAF WT is doublet crossover and, in patients not previously treated with anti-EGFR MoAbs, the addition of cetuximab or panitumumab [II, C].
-
For dMMR/MSI-H tumors progressing after first-line treatment in RAS and BRAF WT patients, line chemotherapy, ipilimumab plus nivolumab or pembrolizumab is recommended [III, B].
Unresectable disease: third and successive line
Classically, 30–40% of patients with mCRC were eligible to receive third-line therapy. However, this percentage has steadily increased as more treatment options have become available. In this setting, targeted therapies can be classified as histology-tuned (RAS, BRAF, and HER2) and histology-agnostic (MSI and NTRK). Subjects with specific molecular alterations (i.e., RAS WT, BRAF V600E mutant or MSI-H/dMMR) and not exposed to targeted therapy in prior lines (anti-EGFR, anti-BRAF + anti-EGFR, or immunotherapy, respectively), should receive them in third line. Other directed therapies are still deemed experimental and would be preferably used in the setting of clinical trials (Fig. 3).
Trifluridine–tipiracil (TAS-102) and regorafenib are two oral drugs approved in refractory mCRC given their limited, yet statistically significant benefits on PFS and OS as compared to placebo in their respective phase III trials [I, A] [67,68,69,70].
TAS-102 is an oral fluoro-pyrimidine with a favorable toxicity profile. The most common adverse events are neutropenia and leukopenia, although febrile neutropenia is uncommon (< 5%). Neutropenia during the first two courses of treatment has been suggested as a positive predictive factor for response [67, 68]. Recently, a beneficial effect on OS, PFS, and RR, and time to worsening to an ECOG PS of > 2 have been reported for the combination of TAS-102 + bevacizumab versus TAS-102 alone in a phase III trial and should be recommended in the third-line setting, albeit approval for its use in our national healthcare system is pending [I, A] [71]. Regorafenib is a multikinase inhibitor with antiangiogenic, anti-stromal, and anti-tumor activity. The most common grade ≥ 3 adverse events are hand–foot syndrome, hypertension, fatigue, and diarrhea [69, 70]. To overcome tolerance issues, various treatment schedules and dosage modifications have been proposed [72].
Fruquintinib is another oral multikinase inhibitor, already approved in China for refractory mCRC. The final results of the FRESCO-2phase III trial (with both occidental and Asian populations) were presented at ESMO 2022. Median OS and PFS were significantly better with respect to placebo. Most participants in this trial had received prior TAS-102 and/or regorafenib [73].
Re-challenge with anti-EGFR agents is an option for tumors that have responded or stabilized with prior anti-EGFR therapy [74]. In phase II trials, third-line RR is 20–30%, with median PFS of 3–4.5 months, and OS of 5–12 months [III, C]. Detection of mutations in ctDNA by liquid biopsy is key to selecting individuals who are most amenable to this treatment approach, though it has not been approved in our country [75].
Some 3–4% of patients with mCRC present HER2 amplification/overexpression and should be considered for treatment with anti-HER2 targeted agents, despite not having been approved by our national healthcare system. Trastuzumab should be combined with a second HER2 inhibitor (lapatinib, pertuzumab) to achieve significant activity [III, C]. The efficacy of these double inhibition or the antibody–drug conjugate (trastuzumab–deruxtecan) has been demonstrated in phase II trials (ORR 30–52% and median PFS 2.9–8.1 months). [24, 76].
KRAS G12C mutations are detected in 3–4% of mCRC patients. Two oral selective inhibitors (sotorasib and adagrasib) are being tested both in monotherapy and in combination with anti-EGFR MoAbs with promising early results. [77, 78]. Finally, NTRK fusions are present in < 2.5% of patients and enriched in right-sided tumors, MSI-H, and native RAS/BRAF tumors. The preliminary activity of targeted drugs is worth mentioning. Larotrectinib and entrectinib have been approved by the EMA and FDA as agnostics for tumors with NTRK rearrangements, but not by our national healthcare system authorities [79, 80]. ALK and ROS1 fusions are even more uncommon (< 1%) (Table 3).
Recommendations
-
TAS-102 plus bevacizumab [I, A] and TAS-102 or regorafenib [I, A] are recommended for patients pre-treated with fluoro-pyrimidines, oxaliplatin, irinotecan, and biologics.
-
Re-challenge with anti-EGFR MoAbs may be an option in selected cases with no RAS/BRAF mutations in ctDNA [III, C].
-
In HER2-positive tumors, treatment with HER2 dual blockade is recommended as an option [III, C].
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Guevara M, Molinuevo A, Salmeron D, Marcos-Gragera R, Carulla M, et al. Cancer survival in adults in spain: a population-based study of the spanish network of cancer registries (REDECAN). Cancers (Basel). 2022;14(10):2441.
Boland PM, Yurgelun MB, Boland CR. Recent progress in Lynch syndrome and other familial colorectal cancer syndromes. CA Cancer J Clin. 2018;68(3):217–31.
Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B, et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24(6):1207–22.
Dykewicz CA. Centers for disease control and prevention (U.S.); Infectious diseases society of America; American society of blood and marrow transplantation. summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001;33(2):139–44.
Atkin W, Dadswell E, Wooldrage K, Kralj-Hans I, von Wagner C, Edwards R, et al. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet. 2013;381(9873):1194–202.
Spada C, Hassan C, Bellini D, Burling D, Cappello G, Carretero C, et al. Imaging alternatives to colonoscopy: CT colonography and colon capsule. European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline—Update 2020. Endoscopy. 2020;52(12):1127–41.
Duffy MJ, Lamerz R, Haglund C, Nicolini A, Kalousová M, Holubec L, et al. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. 2014;134(11):2513–22.
Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674–84.
Vera R, González-Flores E, Rubio C, Urbano J, Valero Camps M, Ciampi-Dopazo JJ, et al. Multidisciplinary management of liver metastases in patients with colorectal cancer: a consensus of SEOM, AEC, SEOR, SERVEI, and SEMNIM. Clin Transl Oncol. 2020;22(5):647–62.
Amin MB, Greene F, Edge S, Compton CC, Gershenwald JE, Brookland RK et al. AJCC Cancer Staging Manual (ed 8th edition). New York: Springer; 2016.
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–705.
André T, Shiu K, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–18.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13.
Pascual J, Attard G, Bidard FC, Curigliano G, De Mattos-Arruda L, Diehn M, et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision medicine working group. Ann Oncol. 2022;33(8):750–68.
Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up†. Ann Oncol. 2022;S0923–7534(22):04192–8.
Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–34.
Van Cutsem E, Lenz HJ, Köhne CH, Heinemann V, Tejpar S, Melezínek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33(7):692–700.
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–65.
Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomised trials. Ann Oncol. 2017;28(8):1713–29.
Yoshino T, Watanabe J, Shitara K, Yasui H, Ohori H, Shiozawa M, et al. Panitumumab (PAN) plus mFOLFOX6 versus bevacizumab (BEV) plus mFOLFOX6 as first-line treatment in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC): Results from the phase 3 PARADIGM trial. J Clin Oncol. 2022:40(17)
Modest DP, Ricard I, Heinemann V, Hegewisch-Becker S, Schmiegel W, Porschen R, et al. Outcome according to KRAS-, NRAS- and BRAF- mutations as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27:1746–53.
Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N Engl J Med. 2019;381(17):1632–43.
Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(6):738-e746.
Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(11):1491–505.
García-Alfonso P, Saiz-Rodríguez M, Mondéjar R, Salazar J, Páez D, Borobia AM, et al. Consensus of experts from the Spanish Pharmacogenetics and Pharmacogenomics Society and the Spanish Society of Medical Oncology for the genotyping of DPYD in cancer patients who are candidates for treatment with fluoropyrimidines. Clin Transl Oncol. 2022;24(3):483–94.
García-Alfonso P, García-Carbonero R, García-Foncillas J, Pérez-Segura P, Salazar R, Vera R, et al. Update of the recommendations for the determination of biomarkers in colorectal carcinoma: National consensus of the Spanish Society of medical oncology and the Spanish Society of Pathology. Clin Transl Oncol. 2020;22(11):1976–91.
Adam R, Kitano Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol Surg. 2019;3(1):50–6.
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–18.
Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301.
Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64.
Pulitano C, Bodingbauer M, Aldrighetti L, de Jong MC, Castillo F, Schulick RD, et al. Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi-institutional analysis. Ann Surg Oncol. 2011;18(5):1380–8.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–15.
Ayez N, van der Stok EP, de Wilt H, Radema SA, van Hillegersberg R, Roumen RM, et al. Neo-adjuvant chemotherapy followed by surgery versus surgery alone in high-risk patients with resectable colorectal liver metastases: the CHARISMA randomized multicenter clinical trial. BMC Cancer. 2015;26(15):180.
Primrose J, Falk S, Finch-Jones M, Valle J, O’Reilly D, Siriwardena A, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601–11.
Wang ZM, Chen YY, Chen FF, Wang SY, Xiong B. Peri-operative chemotherapy for patients with resectable colorectal hepatic metastasis: A meta-analysis. Eur J Surg Oncol. 2015;41(9):1197–203.
Kanemitsu Y, Shimizu Y, Mizusawa J, Inaba Y, Hamaguchi T, Shida D, et al. Hepatectomy Followed by mFOLFOX6 Versus Hepatectomy Alone for Liver-Only Metastatic Colorectal Cancer (JCOG0603): A Phase II or III Randomized Controlled Trial. J Clin Oncol. 2021;39(34):3789–99.
Gonzalez M, Poncet A, Combescure C, Robert J, Ris HB, Gervaz P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20(2):572–9.
Cao C, Yan TD, Black D, Morris DL. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2009;16(8):2152–65.
Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256–66.
Bolhuis K, Kos M, van Oijen MGH, Swijnenburg RJ, Punt CJA. Conversion strategies with chemotherapy plus targeted agents for colorectal cancer liver-only metastases: a systematic review. Eur J Cancer. 2020;141:225–38.
Qadan M, D’Angelica MI. Complex surgical strategies to improve resectability in borderline-resectable disease. Curr Colorectal Cancer Rep. 2015;11:369–77.
Chun YS, Vauthey JN, Boonsirikamchai P, Maru DM, Kopetz S, Palavecino M, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–444.
Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47.
Gruenberger T, Bridgewater J, Chau I, García Alfonso P, Rivoire M, Mudan S, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26(4):702e8.
Garlipp B, Gibbs P, Hazel G, Jeyarajah R, Martin RCG, Bruns CJ, et al. REsect: blinded assessment of amenability to potentially curative treatment of previously unresectable colorectal cancer liver metastases (CRC LM) after chemotherapy ± radioembolization (SIRT) in the randomized SIRFLOX trial. J Clin Oncol. 2017;35:3532.
Datta J, Narayan RR, Kemeny NE, D’Angelica MI. Role of hepatic artery infusion chemotherapy in treatment of initially unresectable colorectal liver metastases: a review. JAMA Surg. 2019;154(8):768-e76.
Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two stage hepatectomy: a planned strategy to treat unresectable liver tumors. Ann Surg. 2000;232:777–85.
Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–14.
Kanemitsu Y, Shitara K, Mizusawa J, Inaba Y, Hamaguchi T, Shida D, et al. Primary tumor resection plus chemotherapy versus chemotherapy alone for colorectal cancer patients with asymptomatic, synchronous unresectable metastases (JCOG1007; iPACS): a randomized clinical trial. J Clin Oncol. 2021;39:1098–107.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). NCCN.org Colon Cancer Version1.2022.
Pietrantonio F, Cremolini C, Aprile G, Lonardi S, Orlandi A, Mennitto A, et al. Single-agent panitumumab in frail elderly patients with advanced RAS and BRAF wild type colorectal cancer: challenging drug label to light up new hope. Oncologist. 2015;20(11):1261–5.
Lonardi S, Schirripa M, Buggin F, Buggin F, Delliponti L, Bergamo F, et al. First-line FOLFOX plus panitumumab versus 5FU plus panitumumab in RAS-BRAF wild-type metastatic colorectal cancer elderly patients: the PANDA study. J Clin Oncol. 2020;38((15_suppl)):4002.
Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14(11):1077–85.
Khattak MA, Martin H, Davidson A, Phillips M. Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: a meta-analysis of randomized clinical trials. Clin Colorectal Cancer. 2015;14(2):81–90.
Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98.
Cremolini C, Antoniotti C, Stein A, Bendell J, Gruenberger T, Rossini D, et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol. 2020;38:3314–24.
Rossini D, Antoniotti C, Lonardi S, Pietrantonio F, Moretto R, Antonuzzo L, et al. Upfront modified fluorouracil, leucovorin, oxaliplatin, and irinotecan plus panitumumab versus fluorouracil, leucovorin, and oxaliplatin plus panitumumab for patients with RAS/BRAF wild-type metastatic colorectal cancer: the phase III TRIPLETE study by GONO. J Clin Oncol. 2022;40(25):2878–88.
Sonbol MB, Mountjoy LJ, Firwana B, Liu AJ, Almader-Douglas D, Mody K, et al. The role of maintenance strategies in metastatic colorectal cancer a systematic review and network meta-analysis of randomized clinical trials. JAMA Oncol. 2020;6:e194489.
Yuan M, Wang Z, Lv W, Pan H. The role of anti-EGFR monoclonal antibody in mCRC maintenance therapy. Front Mol Biosci. 2022;30(9):870395.
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern cooperative oncology group study E3200. J Clin Oncol. 2007;25(1539–44):25.
Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–535.
Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after frst progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37.
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, et al. EPIC: phase III trial of cetuximab plus irinotecan after fuoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–9.
Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fuorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–13.
André T, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann Oncol. 2022;33:1052–60.
Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Eng J Med. 2015;372:1909–19.
Xu J, Kim TW, Shen L, Sriuranpong V, Pan H, Xu R, et al. Results of a randomized, double-blind, placebo-controlled, phase III of Trifluridine/Tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol. 2018;36:350–8.
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12.
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–29.
Tabernero J, Prager GW, Fakih M, Ciardiello F, Van Cutsem E, Elez E, et al. Trifluridine/tipiracil plus bevacizumab for third-line treatment of refractory metastatic colorectal cancer: The phase III randomized Sunlight study. J Clin Oncol. 2023;41(4):4.
Bekaii-Saab TS, Ou FS, Ahn DH, Boland PM, Ciombor KK, Heying EN, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(8):1070–82.
Dasari NA, Lonardi S, Garcia-Carbonero R, Elez ME, Yoshino T, Sobrero AF, et al. FRESCO-2: A global phase III multiregional clinical trial (MRCT) evaluating the efficacy and safety of fruquintinib in patients with refractory metastatic colorectal cancer. Ann Oncol. 2022;33(7):S808–69.
Cremolini C, Rossini D, Dell’Aquila E, Lonardi S, Conca E, Del Re M, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019;5:343–50.
Sartore-Bianchi A, Pietrantonio F, Lonardi S, Mussolin B, Rua F, Crisafulli G, et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat Med. 2022;28(8):1612–8.
Siena S, di Bartolomeo M, Raghav K, Masuishi T, Loupakis F, Kawakami H, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22(6):779–89.
Fakih MG, Kopetz S, Kuboki Y, Kim TW, Munster PN, Krauss JC, et al. Sotorasib for previously treated colorectal cancers with KRASG12C mutation (CodeBreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022;23(1):115–24.
Yaeger R, Weiss J, Pelster MS, Spira AI, Barve M, Ou SI, et al. Adagrasib with or without Cetuximab in colorectal cancer with mutated KRAS G12C. N Engl J Med. 2023;388(1):44–54.
Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. 2020 Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):271–82. https://doi.org/10.1016/S1470-2045(19)30691-6.
Boni V, Drilon A, Deeken J, Leyvraz S, Liu Y, Patel JD, Rosen L, et al. 2021 Efficacy and safety of larotrectinib in patients with tropomyosin receptor kinase fusion-positive gastrointestinal cancer: an expanded dataset. Ann Oncol. 2021;32:S214–5.
Acknowledgements
The authors thank Javier Gallego Plazas and Begoña Graña Suárez for their review and validation of the levels of evidence and grades of recommendation in this guideline.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AFM reports Advisory Board from Amgen; Speaker from Pirre Fabre and Servier; Advisory Board and Speaker from Sanofi and Bayer. VA reports Advisory Board and Speaker from Merck; Advisory Board from Amgen; Speaker from Servier and Sanofi. EE reports Advisory Board, Personal Feels and Other from Amgen, Advisory Board and Personal Feels from Bayer, Hoffman La Roche, Merck, MSD, Pierre Fabre, Sanofi and Servier; Speaker and Personal Feels from Novartis and Organon; Personal Feels and Other from Seagen Intrantional; Other from Array Biopharma, AstraZeneca, Beigene and Boehringer Ingelheim. CG reports Advisory Board from Amgen; Advisory Board and Speaker from Servier; Speaker from Merck and Pierre Fabre. JM reports Grant from ISCIII and Guardant. RVG reports Advisory Board and Speaker from Amgen, Roche and MSD; Speaker from Lilly, Merck and AstraZeneca; Advisory Board, Speaker and Other from Servier and Pierre Fabre. RVT reports Speaker, educational and scientific activities and travel support from Amgen, Servier, BMS and Roche; Speaker from Merck, Sanofi and Bayer and educational and scientific activities from Pierre Fabre and Lilly. JA reports Advisory Board and Speaker from Amgen, Bayer, Pierre Fabre and Servier; Speaker from Merck and MSD. PGA and EA have nothing to disclose.
Ethical approval (Research involving human participants and/or animals) and Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernández Montes, A., Alonso, V., Aranda, E. et al. SEOM-GEMCAD-TTD clinical guidelines for the systemic treatment of metastatic colorectal cancer (2022). Clin Transl Oncol 25, 2718–2731 (2023). https://doi.org/10.1007/s12094-023-03199-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03199-1