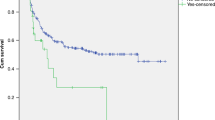
Abstract
Objectives
To investigate the clinical features of thrombotic microangiopathy associated with allogeneic hematopoietic stem cell transplantation in children.
Methods
A retrospective analysis of continuous clinical data from HSCT received in the Department of Hematology and Oncology of Wuhan Children's Hospital from August 1, 2016 to December 31, 2021.
Results
During this period, 209 patients received allo-HSCT in our department, 20 (9.6%) of whom developed TA-TMA. TA-TMA was diagnosed at a median of 94 (7–289) days post-HSCT. Eleven (55%) patients had early TA-TMA within 100 days post-HSCT, while the other 9 (45%) patients had TA-TMA thereafter. The most common symptom of TA-TMA was ecchymosis (55%), while the main signs were refractory hypertension (90%) and multi-cavity effusion (35%). Five (25%) patients had central nervous system symptoms (convulsions and lethargy). All 20 patients had progressive thrombocytopenia, with 16 patients receiving transfusion of platelets that was ineffective. Ruptured red blood cells were visible in only two patients with peripheral blood smears. Cyclosporine A or Tacrolimus (CNI) dose was reduced once TA-TMA was diagnosed. Nineteen cases were treated with low-molecular-weight heparin, 17 patients received plasma exchange, and 12 patients were treated with rituximab. TA-TMA-related mortality percentage in this study was 45% (9/20).
Conclusion
Platelet decline and/or ineffective transfusion post-HSCT should be considered an early indicator of TA-TMA in pediatric patients. TA-TMA in pediatric patients may occur without evidence of peripheral blood schistocytes. Aggressive treatment is required once diagnosis is confirmed, but the long-term prognosis is poor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the most effective curative therapy for several hematological malignancies and bone marrow failure disease in pediatric patients. However, transplantation-related complications provide a serious threat to the long-term survival of patients, one of which is transplantation-associated thrombotic microangiopathy (TA-TMA). TA-TMA is characterized by microvascular hemolytic anemia and thrombocytopenia [1]. The fundamental trigger to onset of TA-TMA is vascular endothelial injury. In addition to the widening of the sub-endothelial space and platelet thrombosis, multiple organ dysfunction also tends to occur particularly of the kidney, heart, intestine, lungs, and brain [2]. Currently, the diagnosis and treatment of TA-TMA in pediatric patients is still in the exploratory stage. In this paper, we retrospectively analyzed the clinical characteristics of 20 pediatric patients with TA-TMA, and hereby present the diagnosis, clinical sequelae, and outcomes.
Methods
Study design, patients, and treatment
This study retrospectively analyzed 20 patients who underwent allogeneic HSCT and were confirmed as having TA-TMA in the Department of Hematology and Oncology, Wuhan Children's Hospital from August 2016 to December 2021. TA-TMA was diagnosed according to the criteria proposed by the Hematopoietic Stem Cell Application Group, Chinese Society of Hematology, Chinese Medical Association [3]. The criteria for inclusion were lactate dehydrogenase (LDH) levels increased to upper normal limit, proteinuria, hypertension [4], thrombocytopenia < 50 × 109/L or a 50% decrease in platelet count, decreased hemoglobin, evidence of microangiopathy, and plasma sC5b-9 values above the upper limit of normal (252 ng/mL) [5]. If 3 of the 7 laboratory or clinical indicators were met, or if tissue biopsy indicators had evidence of microthrombosis, then a diagnosis of TA-TMA was confirmed.
Once diagnosis of TA-TMA was established, the dose of cyclosporin and tacrolimus (CNI) was immediately reduced. Plasma exchange, rituximab, low molecular weight heparin, calcium, defibrotide, eculizumab, and other supportive treatments were administered.
Follow-up endpoints and laboratory metrics
The follow-up endpoint was either June 30, 2022, or patient death. This was achieved by reviewing hospital medical records, laboratory data, conducting telephone interviews, and outpatient reviews. Clinical indicators included clinical manifestations, routine blood tests, platelet transfusion refractoriness (PTR), proteinuria, ruptured red blood cells in peripheral blood, LDH, soluble terminal complement complex sC5b-9, and pathological diagnosis.
Definition
We define a platelet transfusion–refractory patient as an individual who has received two recent (but not necessarily consecutive) platelet transfusions within a 24 h window, and where corrected count increment (CCI) was lower than 4500/μl [6]. We define positive urine protein as random urine protein exceeding the upper limit of normal value3. We use the urine analysis test strip (dry chemical method) of SYSMEX Co., Ltd. to detect urine protein.
Statistical analysis
Statistical analyses were performed using SPSS, version 24.0 (IBM, Chicago, IL). Continuous variables were expressed as median and range, and the categorical variable was expressed as count with percentage. Hierarchical cluster analysis was used in the clustering.
Results
Clinical data
A total of 209 pediatric patients underwent allo-HSCT, of which 20 were diagnosed with TA-TMA. This equates to 9.60% (95% CI = 5.5%–13.6%). The gender split was 11 males (55%) and 9 females (45%), with a median age of 146 (10–190) months. Basic clinical and demographic information for the 20 patients with TA-TMA is shown in Table 1. Primary diseases included aplastic anemia (30%), acute myeloid leukemia (20%), hemophagocytic lymphohistiocytosis (15%) acute lymphoblastic leukemia (5%), Fanconi anemia (5%), myelodysplastic syndrome (5%), mixed-phenotype acute leukemia (5%), mucopolysaccharidosis (5%), and myeloid sarcoma (5%). According to routine clinical practice in our center, haplo-identical donors were a common source of stem cells, with peripheral blood being the main source. The majority of recipients received busulfan (BU)-cyclophosphamide (CY) and anti-thymocyte globulin (ATG) therapy for myeloablative conditioning. Almost all transplant recipients received CNI-based GVHD prophylaxis, and only one case used sirolimus. Nineteen patients (95%) achieved neutrophil and platelet engraftment. The median neutrophil engraftment time was 15 (10–22) days and the median platelet engraftment time was 16 (7–39) days following allo-HSCT. The median count of graft mononuclear cells was 8.335 (0.821–39.6) × 108/kg and the CD34 + cell count was 7.95 (0.353–23.6) × 106/kg.
Clinical features of pediatric patients with TA-TMA
TA-TMA was diagnosed at a median of 94 days (7–289) post-HSCT. Eleven (55%) patients had early-onset TA-TMA, which happened within 100 days post-HSCT, while the remaining 9 (45%) patients had late-onset TA-TMA, which happened at > 100 days post-HSCT. Early-onset TA-TMA occurred at a median of 56 (7–94) days, and late-onset TA-TMA occurred at a median of 157 (104–297) days. Nine cases were combined stage III-IV-GVHD, of which 6 were late-onset TA-TMA. The most common symptom of TA-TMA was ecchymosis. Seventeen cases had varying degrees of bleeding, mainly in the skin and mucous membranes, with black-purple petechiae as a typical manifestation, while the gastrointestinal tract and urinary system were also involved. Case "5" showed diffuse alveolar hemorrhage. Seven cases suffered from dyspnea, which was manifested as high-flow oxygen inhalation of 3 L/min and above mask oxygen inhalation, CAPA-assisted ventilation, and even mechanical ventilation with other advanced support, where transcutaneous oxygen saturation could be maintained at approximately 95%. Five cases had central nervous system symptoms, which manifested as convulsions and lethargy. The main clinical signs of TA-TMA were hypertension and effusion. The blood pressure of 18 cases was higher than the higher blood pressure values typically observed in children with hypertension of the same age [4]. Furthermore, seven cases had serous cavity effusion, mainly manifesting as pleural effusion and pericardial effusion (Table 2).
The median platelet counts, hemoglobin, and LDH levels were 21 (1–79) × 109/L, 79 (41–106)g/L, and 715 (350–4034) U/L, respectively. All 20 patients had a progressive decrease in platelets, of which 16 patients received transfusion that was ineffective. Proteinuria was positive in 16 cases and 2 patients showed ruptured red blood cells in peripheral blood smears. Fifteen patients were monitored for sC5b-9, of which 13 patients displayed higher levels than the normal range. Pathological examination was performed in 13 cases, and microthrombosis was evident in the microvascular pathological films of 7 of them .
Treatment and outcomes
The dose of CNI was reduced once TA-TMA was formally diagnosed. Nineteen patients were treated with low-molecular-weight heparin, 17 patients were treated with plasma exchange, and the median number of plasma exchanges was 4 (3–18). Rituximab was used in 12 patients with a median of 1.5 (1–3) administrations. Two patients were treated with eculizumab and one was treated with defibrotide.
Among the 20 patients, 11 responded well to treatment, with hemoglobin and platelets both increasing, LDH levels and blood pressure decreasing, and no new ecchymosis or petechiae on the skin. Nine patients had a poor response to treatment and unfortunately died (45% mortality from diagnosis). The median time of death was 16 (5–77) days after diagnosis, and the main cause of death was disseminated intravascular coagulation and multi-organ failure. Of the 11 patients who were effectively treated, 4 eventually died of sepsis and acute respiratory failure within 136, 177, 279, and 602 days after diagnosis, respectively. Only seven patients survived to the end of the follow-up period and were reported to be healthy (Table 3).
Discussion
TA-TMA is a serious complication following hematopoietic stem cell transplantation, with incidence varying widely from 0.5% to 76% [7]. In recent years, as the number of patients with TA-TMA has increased, Epperla [8] concluded that the 1-year incidence in a single center was highest at 12% (95% CI = 9%–15%), while the incidence of the disease in the first year after transplantation in our center was slightly lower at 9.60% (95% CI = 5.5%–13.6%).
The onset time of TMA was approximately 27 days and 303 days after transplantation [9], stratified by early-onset TA-TMA and late-onset TA-TMA, respectively. The time of onset of TA-TMA patients in our center was also distributed in two groups, with a similar number of patients with early-onset and late-onset, and 6 of the 9 late-onset patients (66.6%) had combined grade III–IV°GVHD while 3 of the 11 early-onset patients(27.3%). The differences did not reach statistical significance, potentially due to the limited sample size of this small cohort. For patients receiving long-term, continuous use of cyclosporine and tacrolimus, clinicians need to be alert to the occurrence of late-onset TA-TMA, as these appear to be risk factors for TA-TMA. This conclusion needs to be confirmed by studies with larger sample sizes.
The existing diagnostic criteria [7, 10, 11] have been continuously improved in the last few years. In 2014, sC5b-9 detection was incorporated into the TA-TMA diagnostic criteria [12]. In our study, sC5b-9 was monitored in 15 cases, of which 13 cases had levels exceeding the normal range, while the other two cases were elevated before the diagnosis of TA-TMA. A single sC5b-9 value may not corroborate diagnosis, and iterative testing of sC5b-9 may be instructive in the diagnosis and treatment of TA-TMA.
Schistocytes visible on peripheral blood smears were included in multiple diagnostic criteria. However, one case was reported without peripheral blood schistocytes but with evidence of microthrombosis in pathological examinations of the tissue [13]. It should be noted that expert consensus across China has broader diagnostic criteria [3], with pathological diagnosis or the presence of 5 of the 7 markers being the minimum required for diagnosis. Dvorak [14] believes that elevated LDH, proteinuria, and hypertension may be enough for consideration of TA-TMA diagnosis. In our study, only two patients had peripheral blood schistocytes, which is similar to Young [15] who proposed that peripheral blood schistocytes were not an early predictor of TA-TMA.
Fifteen patients had early clinical manifestations such as platelet transfusion without therapeutic response, skin petechiae and ecchymosis. Platelet monitoring is a routine blood test for pediatric patients within 1 year of transplantation. The appearance of ecchymosis and petechiae is more intuitive than that of proteinuria and hypertension. Unexplained platelet transfusion ineffectiveness and new petechiae may emerge as one of the early diagnostic indicators for the diagnosis of TA-TMA. However, the number of cases in the current study is small, which still needs to be verified by more centers in order to confirm sensitivity and specificity for TA-TMA.
Withdrawal of the calcineurin inhibitors such as cyclosporine and tacrolimus seems to be involved in the treatment of TA-TMA [16], but patients with GVHD need to be evaluated for replacement or reduction of anti-rejection drugs. Plasma exchange is one of the most common methods for the treatment of patients with thrombotic thrombocytopenic purpura at present, but it has poor efficacy in TA-TMA [17]. In recent years, Jodele demonstrated through a retrospective study that early use of plasma exchange may be beneficial for patients with multiple organ failure caused by TA-TMA [18]. Yang [19] has shown that plasma exchange was effective in TA-TMA patients without gastrointestinal bleeding, and it is possible that the frequency and duration of plasma exchange might achieve better efficacy. Plasma exchange was used in 17 patients in this study, where clinical symptoms improved and disease progression slowed down. However, only six patients survived. In particular, the effect of plasma exchange was not good in patients with bleeding.
McFadyen [20] has proposed that endothelial cell damage caused by an immune response to infection could resolve microthrombosis through an anticoagulant pathway. The pathogenesis of TA-TMA is also influenced by the development of microthrombi caused by endothelial injury. Tissue plasminogen activator and low-molecular-weight heparin may be future treatments for TA-TMA. Low-molecular-weight heparin-calcium infusion was used in 19 patients, but its exact efficacy was unclear due to the combination of other treatments. Rituximab is a CD20 monoclonal antibody whose role in TA-TMA is unclear. In the review by Kim [21], 12 of 15 patients (80%) who received rituximab resulted in therapeutic efficacy. In our study, twelve patients were treated with rituximab, of which four experienced ineffective response. However, rituximab is often used in combination with plasma exchange, and the exact effect is not clear in TA-TMA. Defibrotide has been shown to exhibit antithrombotic and fibrinolytic activities. Higham [22] demonstrated that defibrotide may reduce the risk of TA-TMA in pediatric patients. One patient in our center was given defibrotide but it did not work. Eculizumab is a recombinant human monoclonal antibody that inhibits terminal complement C5. The antibody has high affinity for C5, blocks the formation of C5a and C5b-9, and protects vascular endothelial cells from C5b-9-mediated damage. In a study of 64 pediatric patients with high-risk TA-TMA [23], 36 (56%) patients treated with eculizumab achieved complete remission and 5 (8%) achieved a partial response. The remaining 23 (36%) had no response at the end of treatment. Eculizumab improved 1-year post-HSCT survival. Two patients in our center were treated with eculizumab and both achieved remission, but both patients also developed serious infections after its use. Further research is needed for the prevention of treatment complications arising from eculizumab.
In conclusion, TA-TMA is a serious complication after allo-HSCT, with a high mortality rate. Early identification and prompt treatment are key to saving lives. TA-TMA in pediatric patients may occur without evidence of peripheral blood schistocytes. Unexplained precipitous thrombocytopenia or ineffective platelet transfusions may be the earliest signs of TA-TMA in pediatric patients and could be early diagnostic indicators. However, multi-center studies are needed to provide sufficient patient numbers in confirming our observations.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Rosenthal J. Hematopoietic cell transplantation-associated thrombotic microangiopathy: a review of pathophysiology, diagnosis, and treatment. J Blood Med. 2016;7:181–6. https://doi.org/10.2147/jbm.S102235.
Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118(6):1452–62. https://doi.org/10.1182/blood-2011-02-321315.
[Chinese consensus on the diagnosis and management of transplant-associated thrombotic microangiopathy (2021)]. Zhonghua Xue Ye Xue Za Zhi. Mar 14 2021;42(3):177–184. doi:https://doi.org/10.3760/cma.j.issn.0253-2727.2021.03.001
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. Sep 2017;140(3). doi:https://doi.org/10.1542/peds.2017-1904
Horváth O, Kállay K, Csuka D, Mező B, Sinkovits G, Kassa C, et al. Early increase in complement terminal pathway activation marker sC5b-9 is predictive for the development of thrombotic microangiopathy after Stem Cell Transplantation. Biol Blood Marrow Transplant. 2018;24(5):989–96. https://doi.org/10.1016/j.bbmt.2018.01.009.
Matsui R, Hagino T, Tsuno NH, Ohtani H, Azuma F, Matsuhashi M, et al. Does time of CCI measurement affect the evaluation of platelet transfusion effectiveness? Transfus Apher Sci. 2021;60(3):103–23. https://doi.org/10.1016/j.transci.2021.103123.
Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571–5. https://doi.org/10.1016/j.bbmt.2005.06.001.
Epperla N, Li A, Logan B, Fretham C, Chhabra S, Aljurf M, et al. Incidence, risk factors for and outcomes of transplant-associated thrombotic microangiopathy. Br J Haematol. 2020;189(6):1171–81. https://doi.org/10.1111/bjh.16457.
Heybeli C, Sridharan M, Alkhateeb HB, Villasboas Bisneto JC, Buadi FK, Chen D, et al. Characteristics of late transplant-associated thrombotic microangiopathy in patients who underwent allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2020; doi:https://doi.org/10.1002/ajh.25922
Ruutu T, Barosi G, Benjamin RJ, Clark RE, George JN, Gratwohl A, et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92(1):95–100. https://doi.org/10.3324/haematol.10699.
Cho BS, Yahng SA, Lee SE, Eom KS, Kim YJ, Kim HJ, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90(8):918–26. https://doi.org/10.1097/TP.0b013e3181f24e8d.
Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124(4):645–53. https://doi.org/10.1182/blood-2014-03-564997.
Wirtschafter E, VanBeek C, Linhares Y. Bone marrow transplant-associated thrombotic microangiopathy without peripheral blood schistocytes: a case report and review of the literature. Exp Hematol Oncol. 2018;7:14. https://doi.org/10.1186/s40164-018-0106-9.
Dvorak CC, Higham C, Shimano KA. Transplant-associated thrombotic microangiopathy in pediatric hematopoietic cell transplant recipients: a practical approach to diagnosis and management. Front Pediatr. 2019;7:133. https://doi.org/10.3389/fped.2019.00133.
Young JA, Pallas CR, Knovich MA. Transplant-associated thrombotic microangiopathy: theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transplant. 2021;56(8):1805–17. https://doi.org/10.1038/s41409-021-01283-0.
Seaby EG, Gilbert RD. Thrombotic microangiopathy following haematopoietic stem cell transplant. Pediatr Nephrol. 2018;33(9):1489–500. https://doi.org/10.1007/s00467-017-3803-4.
Mulay S, Kreuter JD, Bryant SC, Elliott MA, Hogan WJ, Winters JL, et al. Outcomes of plasma exchange in patients with transplant-associated thrombotic microangiopathy based on time of presentation since transplant. J Clin Apher. 2015;30(3):147–53. https://doi.org/10.1002/jca.21352.
Jodele S, Laskin BL, Goebel J, Khoury JC, Pinkard SL, Carey PM, et al. Does early initiation of therapeutic plasma exchange improve outcome in pediatric stem cell transplant-associated thrombotic microangiopathy? Transfusion. 2013;53(3):661–7. https://doi.org/10.1111/j.1537-2995.2012.03776.x.
Yang LP, Zhao P, Wu YJ, Fu HX, He Y, Mo XD, et al. Treatment outcome and efficacy of therapeutic plasma exchange for transplant-associated thrombotic microangiopathy in a large real-world cohort study. Bone Marrow Transplant. 2022;57(4):554–61. https://doi.org/10.1038/s41409-022-01581-1.
McFadyen JD, Stevens H, Peter K. The emerging threat of (Micro)Ttrombosis in COVID-19 and its therapeutic implications. Circ Res. 2020;127(4):571–87. https://doi.org/10.1161/circresaha.120.317447.
Kim SS, Patel M, Yum K, Keyzner A. Hematopoietic stem cell transplant-associated thrombotic microangiopathy: review of pharmacologic treatment options. Transfusion. 2015;55(2):452–8. https://doi.org/10.1111/trf.12859.
Higham CS, Shimano KA, Melton A, Kharbanda S, Chu J, Dara J, et al. A pilot trial of prophylactic defibrotide to prevent serious thrombotic microangiopathy in high-risk pediatric patients. Pediatr Blood Cancer. 2022;69(5):e29641. doi:https://doi.org/10.1002/pbc.29641
Jodele S, Dandoy C, Lane A, Laskin B, Teusink-Cross A, Myers K, et al. Complement blockade for TA-TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood. 2020;135(13):1049–57. https://doi.org/10.1182/blood.2019004218.
Acknowledgements
The authors thank the participating patients and their caregivers, and the study centers and investigators for their contributions to the study. This study was funded by Wuhan Clinical Medical Research Project (WX20D20, WZ20Y04, WX21Z48).
Funding
Wuhan Clinical Medical Research Project, WX20D20, Li Yang, WZ20Y04, Zhi Chen, WX21Z48, Zhuo Wang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics statement
Ethical approval was obtained from the Ethics Committee of Wuhan Children's Hospital (2022R017-E01).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed consent statement
The study was done after agreement from the local ethics committee and written informed consent was obtained from all participants and/or their guardians.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, L., Xiong, H., Chen, Z. et al. Clinical characteristics of pediatric allogeneic hematopoietic stem cell transplantation-associated thrombotic microangiopathy (TA-TMA): a retrospective single-center analysis. Clin Transl Oncol 25, 2451–2461 (2023). https://doi.org/10.1007/s12094-023-03129-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-023-03129-1