Abstract
Purpose
Triple-negative breast cancer (TNBC) is characterized by large heterogeneity and relative lack of available targeted therapies. To find therapeutic strategies for distinct patients with TNBC, several approaches have been used for TNBC clustering, including recently immune and phosphoproteomic patterns. Based on 70-kDa ribosomal protein S6 kinase (P70S6K)-TNBC clustering, the current study explores the immune profiling in TNBC tumors.
Methods
Stromal tumor-infiltrating lymphocytes (sTILs) were evaluated in human TNBC tumor samples. Furthermore, immunohistochemistry staining for CD8, CD4, Foxp3, and CD20 was performed in tissue microarrays (TMA) sections.
Results
Histological analysis showed decreased sTILs, CD20+ cells, and CD8+/CD4+ ratio in high phosphorylated P70S6K (p-P70S6K) tumors. Moreover, p-P70S6K score was directly correlated with CD4+ and Foxp3+ T cells, while it was inversely correlated with CD8+/CD4+ and CD8+/Foxp3+ ratios.
Conclusion
sTIL infiltration and lymphocyte profiling vary in the context of hyperactivation of P70S6K in TNBC tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancer (TNBC) is an aggressive breast cancer (BC) subtype frequently associated to rapid progression and high rate of early recurrences and metastasis in brain, liver, and lung [1,2,3,4,5]. Due to its poor differentiation and molecular heterogeneity, TNBC is challenging to treat and responses to treatment are frequently short-lasting.
Several approaches based on gene expression patterns, transcriptomic profiling and other strategies have been implemented for TNBC clustering. Lately, immunogenic profile has been used to classify TNBC tumors, which have shown the highest immune infiltration among BC subtypes [6, 7]. A recent study has confirmed the prognostic role of tumor-infiltrating lymphocytes (TILs) in TNBC [8]. However, some challenges remain in establishing the analytical and clinical validity of TILs in BC.
Trying to identify new targets that allow stratification of TNBC, we have previously described a functional clustering based on kinase activity profiling [9]. One of the kinases described was the 70-kDa ribosomal protein S6 kinase (P70S6K) whose hyperactivation was associated with adverse outcome [9]. P70S6K is a serine/threonine kinase triggered downstream of the PI3K/AKT/mTOR pathway, which is often activated in patients with TNBC [10,11,12,13]. P70S6K is overexpressed in cell lines and TNBC clinical samples and it has been proposed as a mediator of cancer cell migration, invasion, and metastasis [14,15,16,17,18].
Most of TNBC clustering approaches are based on a single strategy; however, given the heterogeneity of this BC subtype, combined information from different clustering patterns could provide a more rational stratification system. The present study relies on P70S6K profiling to characterize the immune signature of patients with TNBC.
Material and methods
Tumor samples
Female patients with a diagnosis of primary, non-metastatic BC, with expression of estrogen receptor (ER) and/or progesterone receptor (PR) < 1% and lack of human epidermal growth factor receptor 2 (HER2) amplification diagnosed at Hospital 12 de Octubre, Hospital de Fuenlabrada and Hospital La Princesa were eligible for this study. HER2 amplification was defined as a HER2/CEP17 ratio > 2 in FISH or a value of +++/+++ in an HERCEPTEST. Samples were obtained before the administration of any treatment.
Immunohistochemistry analysis
Tumor samples were included in fresh 10% neutral buffered formalin immediately after sample collection. TMA were mounted with two 1.5 mm cores per sample. Deparaffinization and antigen retrieval were performed in an automated immunostaining platform (Discovery XT-ULTRA, Leica) using validated reagents (Ventana, Roche). The following antibodies were used: phospho-p70S6K (Thr389) (1A5); CD8 (C8/144B); CD4 (4B12); Foxp3 (236A/E7); CD20 (L26). Whole slides were acquired with a slide scanner (AxioScan Z1, Zeiss). Digital images were analyzed with image analysis system Zen Blue Software (V 3.1, Zeiss). In case of p-P70S6K staining, an H-score was generated as described previously [9]. For normalized data, a Z-score of p-P70S6K was calculated for each hospital and staining run. For immune infiltrate characterization, percentage of each lymphocyte marker was calculated as total positive area normalized to total area. Regarding TILs quantification, percentage of stromal TILs (sTILs) was evaluated from hematoxylin and eosin (H&E) stained sections of tumor samples according to the International Immuno-Oncology Working Group Guidelines [19,20,21,22].
Statistics
Statistical analyses were performed using Prism (Prism 8.3.0, GraphPad software). Differences between groups were determined using unpaired two-tailed Student’s t test. Correlation analyses were performed using Spearman’s test.
Results
To characterize the tumor lymphocyte infiltrate in the context of hyperactivation of P70S6K, TILs quantification and profiling were performed in primary samples of 341 patients with TNBC. Demographic information and characteristics of patients with TNBC are presented in Table 1.
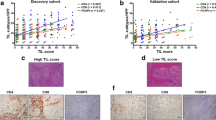
Phosphorylation levels of P70S6K were determined by immunohistochemistry staining as previously published [9]. H-Score of p-P70S6K expression was calculated using an image analysis software and samples were classified as high p-P70S6K (herein “pP70-High”; including samples in the upper quartile) or low p-P70S6K (herein “pP70-Low”; the remaining patients). Photomicrographs of representative stained tissue samples and analysis are shown (Fig. 1A, B). Tumor immune infiltration was studied by evaluation of sTILs (Fig. 1C). Percentage range of sTILs (0%, 1–9%, 10–20%, > 20%) and p-P70S6K status distributions according to clinical characteristic of patients are shown in Table 2. Changes in tumor infiltration were found between both types of patients as sTILs percentage showed a significant decrease in those patients exhibiting hyperactivation of P70S6K (12.40 ± 1.965 pP70-High; 17.70 ± 1.574 pP70-Low) (Fig. 1D).
Microscopic evaluation of p-P70S6K and tumor-infiltrating lymphocytes in patients with TNBC. A Representative immunohistochemistry images of pP70-High (left) and pP70-Low (right) from TMA cores of TNBC tumor samples. Bar indicates 100 µm. B Representative images of p-P70S6K staining analysis. H-Score was generated as previously published [9] considering areas of high staining (red, Area_Color 1), medium staining (green, Area_Color 2), and low staining (blue, Area_Color 3). Percentage of each staining per sample was normalized to total area, including the negative-staining area (yellow), providing a computerized H-score calculated by formula: ((% of Area_Color1 × 3) + (% of Area_Color2 × 2) + (% of Area_Color3 × 1))/100. Images were acquired with a slide scanner (AxioScan Z1, Zeiss) and photomicrographs were obtained using the image analysis system Zen Blue Software (V3.1). C Representative images of H&E staining of pP70-High (left) and pP70-Low (right) TNBC tumor cases. Magnification ×20. Images were acquired with digital microscope Leica DMD 108 from LeicaMicrosystems. D Percentage of sTILs was determined by expert pathologists following recommendation guidelines. Box and Whiskers plots are shown for sTILs percentage in pP70-High (dark) or pP70-Low (grey) specimens. Unpaired two-tailed t test with Welch’s correction was performed and statistically significant P value is shown. n = 82 pP70-High; n = 244 pP70-Low
Given the functional heterogeneity of tumor lymphocytic infiltrate, individual characterization of lymphocyte populations was next performed to add specificity to the value of sTILs estimation. Single immunohistochemical staining of CD8+, CD4+, Foxp3+, and CD20+ cells was used for analysis of cytotoxic T cells, total helper T cells, regulatory T cells (Tregs), and B cells, respectively (Fig. 2A). When comparing pP70-High and pP70-Low cases, a significant decrease in CD20+ cells was seen in high phosphorylated samples (Fig. 2B). However, no significant differences were found in T lymphocyte populations between both types of samples (Fig. 2B).
CD8+ and CD4+ T cells, Tregs and B cells quantification in pP70-High and pP70-Low TNBC. A Representative immunohistochemistry images of an area showing CD8, CD4, Foxp3, and CD20 staining. Bar indicates 50 µm. Images were acquired with a slide scanner (AxioScan Z1, Zeiss) and photomicrographs were obtained using the image analysis system Zen Blue Software (V3.1). B Mean values of percentage of CD8+, CD4+, Foxp3+, and CD20+ (left) and mean values of CD8+/CD4+, CD8+/Foxp3+, and Foxp3+/CD4+ ratios (right) in pP70-High (dark) or pP70-Low (grey) specimens. n = 74–81 pP70-High; n = 213–241 pP70-Low. Data were calculated using an automated scanning microscope (AxioScan Z1, Zeiss) and computerized image analysis system (Zen Blue Software V3.1). Percentage of positive area (intraepithelial and stromal) from each lymphocyte population is referred to total core area. Plots show mean ± SEM. Unpaired two-tailed t test with/without Welch’s correction was performed and statistically significant P values are shown. C Spearman’s correlation between p-P70S6K and each lymphocyte marker and ratio was determined. Individual correlation plots are shown for statistically significant results. Spearman’s coefficient of the correlation between the two markers is shown (R and P values). n = 286–321
As the equilibrium between different T cell subsets is involved in the final outcome of the tumor immune response, CD8+/CD4+, CD8+/Foxp3+, and Foxp3+/CD4+ ratios were calculated. Significant decrease of CD8+/CD4+ ratio was seen in pP70-High samples (Fig. 2B).
Additionally, monotonic relationships between each population and p-P70S6K score were analyzed. While continuous Foxp3 and CD4 expression levels were positively correlated with p-P70S6K status, negative correlations were found for CD8+/CD4+ and CD8+/Foxp3+ ratios (Fig. 2C).
Discussion
The lack of classical histologic biomarkers and the huge heterogeneity of TNBC make the profiling of this BC subtype necessary for defining different treatment strategies. Several approaches have been investigated for TNBC stratification, including lately those based on the prognostic role of TILs to describe distinct response subtypes of TNBC following adjuvant chemotherapy [8]. Recently, we have also described the prognostic impact of hyperactivation of P70S6K and other kinases on disease outcome and risk of relapse of patients with TNBC [9]. Based on our previous study, we decided to investigate the relationship between tumor P70S6K hyperactivation and immune infiltration in TNBC. First, we broadly examined the immune infiltrate by determining sTILs in tumors expressing high or low phosphorylation levels of P70S6K. Results indicate a decreased sTILs percentage in those tumors overexpressing p-P70S6K. Given the association of TILs with good prognosis in TNBC [8], it is predictable that tumor microenvironment (TME) is less infiltrated in P70S6K hyperphosphorylated tumors which have previously shown worse prognosis [9]. Relationships between P70S6K expression in BC and TILs have not yet been clearly defined. A previous study in HER2-positive BC has shown that tumors with high levels of pS6 expression are associated with high percentage of TILs; however, the specific role of P70S6K was not determined [23].
Characterization of tumors as rich- and poor-TILs may not completely replicate distinct immune responses that can be initiated in the TME. Therefore, we further characterized immune subpopulations in our set of TNBC samples. We found significant decrease in CD20+ cells in pP70-High tumors, whereas not significant differences were found in T lymphocytes between both types of samples. However, a positive correlation was found for CD4 and Foxp3 expression and p-P70S6K score.
As different subsets of T cells may act in opposite ways against tumor progression, we also determined balances between them in pP70-High and pP70-Low samples. Analysis of ratios among different populations of T cells has previously demonstrated a great utility in TNBC. Several studies have reported association of CD8/Foxp3, CD8/CD4, and CD4/Foxp3 with improved survival of patients and response to different treatments [24,25,26,27]. However, while increased CD4/CD8 ratio was related with adverse prognosis in triple-negative invasive ductal carcinoma [28], another study has described an association between CD4/CD8 ratio and distant relapse-free survival and overall survival in TNBC [29]. In our cohort of patients with TNBC, a decreased CD8+/CD4+ ratio was seen in pP70-High tumors. Moreover, negative correlations for p-P70S6K score and CD8+/CD4+ and CD8+/Foxp3+ ratios were found. Although correlations do not indicate causation, our findings suggest that P70S6K activity may negatively influence pro-inflammatory CD8 immune responses while favoring regulatory T cell responses. Similarly, a previous study has suggested that deregulation of the PI3K pathway could contribute to immune escape by mitigating pro-inflammatory responses and increasing the percentage of Foxp3+ lymphocytes [30]. More functional studies are needed for a better understanding of precise mechanisms regulating T cell function developed by P70S6K in TNBC. A limitation of our study is that we did not illustrate the mechanisms by which P70S6K modulates the lymphoid landscape in TNBC tumors. Increasing the difficulty, the huge complexity of lymphocytes at both levels, cell phenotype and activation status, constitutes a challenge when studying T cell responses. Our analyses in TNBC patient-derived samples do not inform about specific lymphocyte phenotypes (i.e., different subsets of T helper cells) or their activation status and functionality. Further characterization of phenotypic or activation markers of lymphocytes should be considered when defining lymphocyte infiltrates in TNBC.
In brief, our study describes the lymphocyte profiling in the context of P70S6K hyperactivation in TNBC, providing valuable data to be considered for improved TNBC clustering. While during the last years, phosphoproteomic approaches are gaining attention in BC, how this strategy can be combined with new and promising immune-profiling approaches has to be further explored.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. https://doi.org/10.1158/1078-0432.CCR-06-3045.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. New Engl J Med. 2010;363:1938–48. https://doi.org/10.1056/NEJMra1001389.
Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong Y-N, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–72. https://doi.org/10.1002/cncr.27581.
Li X, Yang J, Peng L, Sahin AA, Huo L, Ward KC, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;161:279–87. https://doi.org/10.1007/s10549-016-4059-6.
Xiao W, Zheng S, Yang A, Zhang X, Zou Y, Tang H, et al. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: a population-based study. Cancer Manag Res. 2018;10:5329–38. https://doi.org/10.2147/CMAR.S176763.
Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2:1354–60. https://doi.org/10.1001/jamaoncol.2016.1061.
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. https://doi.org/10.1016/S1470-2045(17)30904-X.
Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37:559–69. https://doi.org/10.1200/JCO.18.01010.
Zagorac I, Fernandez-Gaitero S, Penning R, Post H, Bueno MJ, Mouron S, et al. In vivo phosphoproteomics reveals kinase activity profiles that predict treatment outcome in triple-negative breast cancer. Nat Commun. 2018;9:3501. https://doi.org/10.1038/s41467-018-05742-z.
Pérez-Tenorio G, Stal O. Southeast Sweden Breast Cancer Group. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002;86:540–5. https://doi.org/10.1038/sj.bjc.6600126.
Sohn J, Do KA, Liu S, Chen H, Mills GB, Hortobagyi GN, et al. Functional proteomics characterization of residual triple-negative breast cancer after standard neoadjuvant chemotherapy. Ann Oncol. 2013;24:2522–6. https://doi.org/10.1093/annonc/mdt248.
Ganesan P, Moulder S, Lee JJ, Janku F, Valero V, Zinner RG, et al. Triple-negative breast cancer patients treated at MD Anderson Cancer Center in phase I trials: improved outcomes with combination chemotherapy and targeted agents. Mol Cancer Ther. 2014;13:3175–84. https://doi.org/10.1158/1535-7163.MCT-14-0358.
Mercatali L, Spadazzi C, Miserocchi G, Liverani C, De Vita A, Bongiovanni A, et al. The effect of everolimus in an in vitro model of triple negative breast cancer and osteoclasts. Int J Mol Sci. 2016;17:1827. https://doi.org/10.3390/ijms17111827.
Akar U, Ozpolat B, Mehta K, Lopez-Berestein G, Zhang D, Ueno NT, et al. Targeting p70S6K prevented lung metastasis in a breast cancer xenograft model. Mol Cancer Ther. 2010;9:1180–7. https://doi.org/10.1158/1535-7163.MCT-09-1025.
Janaki Ramaiah M, Lavanya A, Honarpisheh M, Zarea M, Bhadra U, Bhadra MP. MiR-15/16 complex targets p70S6 kinase 1 and controls cell proliferation in MDA-MB-231 breast cancer cells. Gene. 2014;552:255–64. https://doi.org/10.1016/j.gene.2014.09.052.
Khotskaya YB, Goverdhan A, Shen J, Ponz-Sarvise M, Chang SS, Hsu MC, et al. S6K1 promotes invasiveness of breast cancer cells in a model of metastasis of triple-negative breast cancer. Am J Transl Res. 2014;6:361–76 (eCollection 2014).
Berman AY, Manna S, Schwartz NS, Katz YE, Sun Y, Behrmann CA, et al. ERRα regulates the growth of triple-negative breast cancer cells via S6K1-dependent mechanism. Signal Transduct Target Ther. 2017;2: 217035. https://doi.org/10.1038/sigtrans.2017.35.
Razaviyan J, Hadavi R, Tavakoli R, Kamani F, Paknejad M, Mohammadi-Yeganeh S. Expression of miRNAs targeting mTOR and S6K1 genes of mTOR signaling pathway including miR-96, miR-557, and miR-3182 in triple-negative breast cancer. Appl Biochem Biotechnol. 2018;186:1074–89. https://doi.org/10.1007/s12010-018-2773-8.
Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. https://doi.org/10.1200/JCO.2009.23.7370.
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71. https://doi.org/10.1093/annonc/mdu450.
Denkert C, Wienert S, Poterie A, Loibl S, Budczies J, Badve S, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the International Immuno-Oncology Biomarker Working Group. Mod Pathol. 2016;29:1155–64. https://doi.org/10.1038/modpathol.2016.109.
Dieci MV, Radosevic-Robin N, Fineberg S, van den Eynden G, Ternes N, Penault-Llorca F, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018;52:16–25. https://doi.org/10.1016/j.semcancer.2017.10.003.
Kim JY, Heo SH, Song IH, Park IA, Kim YA, Gong G, et al. Activation of the PERK-eIF2α pathway is associated with tumor-infiltrating lymphocytes in HER2-positive breast cancer. Anticancer Res. 2016;36:2705–11.
Tavares CM, Sampaio CD, Lima GE, Andrade VP, Gonçalves DG, Macedo MP, et al. A high CD8 to FOXP3 ratio in the tumor stroma and expression of PTEN in tumor cells are associated with improved survival in non-metastatic triple-negative breast carcinoma. BMC Cancer. 2021;21:901. https://doi.org/10.1186/s12885-021-08636-4.
Miyashita M, Sasano H, Tamaki K, Chan M, Hirakawa H, Suzuki A, et al. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in triple-negative breast cancer: its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2014;148:525–34. https://doi.org/10.1007/s10549-014-3197-y.
Nabholtz JM, Chalabi N, Radosevic-Robin N, Dauplat MM, Mouret-Reynier MA, Van Praagh I, et al. Multicentric neoadjuvant pilot Phase II study of cetuximab combined with docetaxel in operable triple negative breast cancer. Int J Cancer. 2016;138:2274–80. https://doi.org/10.1002/ijc.29952.
Castaneda CA, Mittendorf E, Casavilca S, Wu Y, Castillo M, Arboleda P, et al. Tumor infiltrating lymphocytes in triple negative breast cancer receiving neoadjuvant chemotherapy. World J Clin Oncol. 2016. https://doi.org/10.5306/wjco.v7.i5.387.
Jafarian AH, Tasbandi A, Gilan H, Sheikhi M, Roshan NM. Evaluation of CD30/CD4/CD8 in triple-negative invasive ductal carcinoma of breast in association with clinicopathological prognostic factors. Indian J Pathol Microbiol. 2018;61:500–4. https://doi.org/10.4103/IJPM.IJPM_67_18.
Wang K, Shen T, Siegal GP, Wei S. The CD4/CD8 ratio of tumor-infiltrating lymphocytes at the tumor-host interface has prognostic value in triple-negative breast cancer. Hum Pathol. 2017;69:110–7. https://doi.org/10.1016/j.humpath.2017.09.012.
Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, et al. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28:306–12. https://doi.org/10.1038/onc.2008.384.
Acknowledgements
We acknowledge the Histopathology Core Unit for technical support in immunohistochemistry studies.
Funding
RJ is a recipient of the following grants: Eva Plaza/CNIO Friends Fellowship and Marie Sklodowska-Curie Individual Fellowship. The project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 893597. RC is a recipient of the ISCIII grants: PI17/01865 and PI20/01458. MQF is a recipient of the following Grants: AES-PI19/00454 funded by the ISCIII and co-funded by the European Regional Development Fund (ERDF) and B2017/BMD3733 (Immunothercan-CM)—Call for Coordinated Research Groups from Madrid Region—Madrid Regional Government—ERDF funds. The study was also funded by CRIS Contra el Cancer Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization was done by RJ and MQF; patients´ sample and data collection was done by RSB, LM, MM, AGG, RSP, and RC; methodology was done by RJ; formal analysis was done by RJ, SM, RS, SL, BPM, and MQF; project administration was done by RJ, SM, and MQF; funding acquisition was done by RJ, RC, and MQF; Writing—original draft was done by RJ; Writing—review and editing was done by RJ and MQF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Instituto de Salud Carlos III (CEI EC1124, 19 December 2017; CEI 11/137, 6 September 2017).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jimeno, R., Mouron, S., Salgado, R. et al. Tumor P70S6K hyperactivation is inversely associated with tumor-infiltrating lymphocytes in triple-negative breast cancer. Clin Transl Oncol 25, 1124–1131 (2023). https://doi.org/10.1007/s12094-022-03006-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-03006-3